Question: please show all work and give general formula(s) before applying them. explanations are appreciated. AB (1) The equilibrium ki A + B ki has been
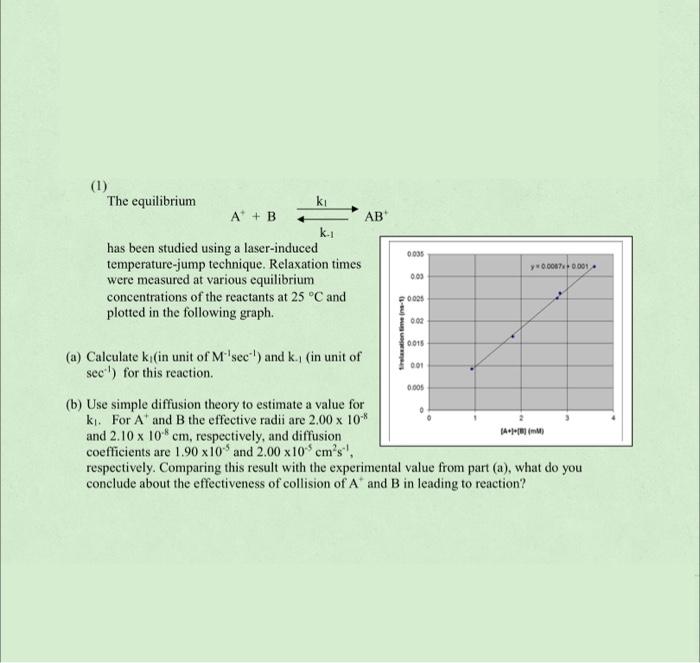
AB (1) The equilibrium ki A + B ki has been studied using a laser-induced temperature-jump technique. Relaxation times were measured at various equilibrium concentrations of the reactants at 25 C and plotted in the following graph. 0035 y 0.008740001. 003 0009 0.02 wonens 0015 001 0.005 (a) Calculate k (in unit of M''sec ) and k. (in unit of sec!) for this reaction. (b) Use simple diffusion theory to estimate a value for k. For A and B the effective radii are 2,00 x 10-* and 2.10 x 10 cm, respectively, and diffusion coefficients are 1.90 x10 and 2.00 x10 cm's! respectively. Comparing this result with the experimental value from part (a), what do you conclude about the effectiveness of collision of A and B in leading to reaction? ( AMM)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


