Question: Please show all work. Don't manipulate the equation. Keep the format in... Ln (P2/P1) = - Hvap/R * (1/T1 - 1/T2) 2. A liquid has
Please show all work. Don't manipulate the equation. Keep the format in...
Ln (P2/P1) = - Hvap/R * (1/T1 - 1/T2)
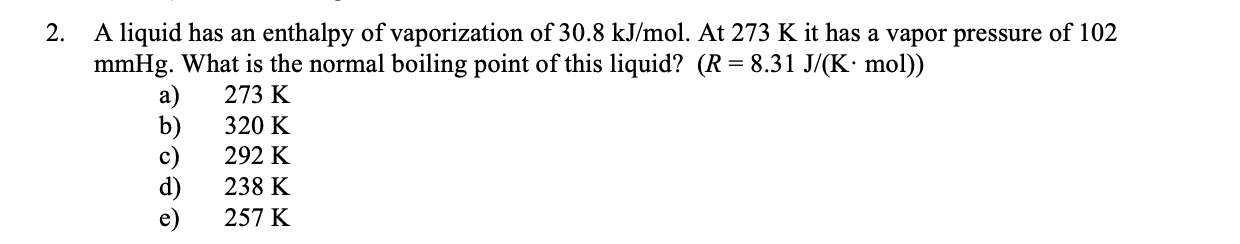
2. A liquid has an enthalpy of vaporization of 30.8kJ/mol. At 273K it has a vapor pressure of 102 mmHg. What is the normal boiling point of this liquid? (R=8.31J/(Kmol)) a) 273K b) 320K c) 292K d) 238K e) 257K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


