Question: Please show all work. Frequently calculations are simplified by using 'atomic units', where we let several constants be (numerically) equal to one. This allows simple
Please show all work.
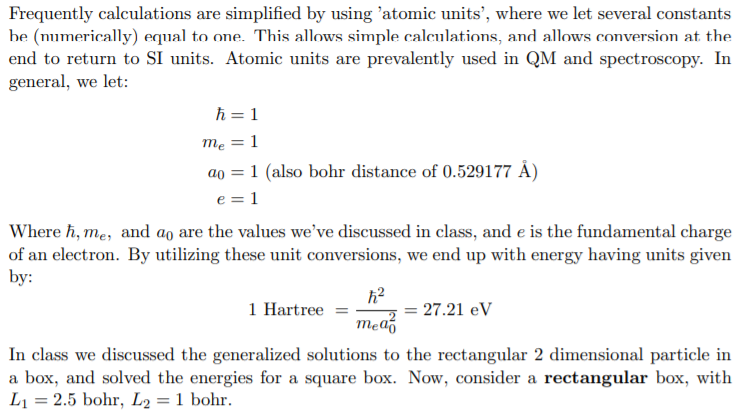

Frequently calculations are simplified by using 'atomic units', where we let several constants be (numerically) equal to one. This allows simple calculations, and allows conversion at the end to return to SI units. Atomic units are prevalently used in QM and spectroscopy. In general, we let: mea0e=1=1=1(alsobohrdistanceof0.529177A)=1 Where ,me, and a0 are the values we've discussed in class, and e is the fundamental charge of an electron. By utilizing these unit conversions, we end up with energy having units given by: 1Hartree=mea022=27.21eV In class we discussed the generalized solutions to the rectangular 2 dimensional particle in a box, and solved the energies for a square box. Now, consider a rectangular box, with L1=2.5 bohr, L2=1 bohr. A) Calculate the first 6 energy levels of an electron in this 2D rectangular box, and plot them as an energy diagram. What do you notice about the differences between those levels and those for a square well (Note: a square well has L1=L2 )? B) Using a 3D graphing application of your choice, e.g. geogebra.org, create a graph of the wavefunction and the probability density for the ground state and first two excited states. (Transfer pictures of your graphs to a PDF document and merge this PDF with your primary solution document, make sure to select your graphs within gradescope during submission.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


