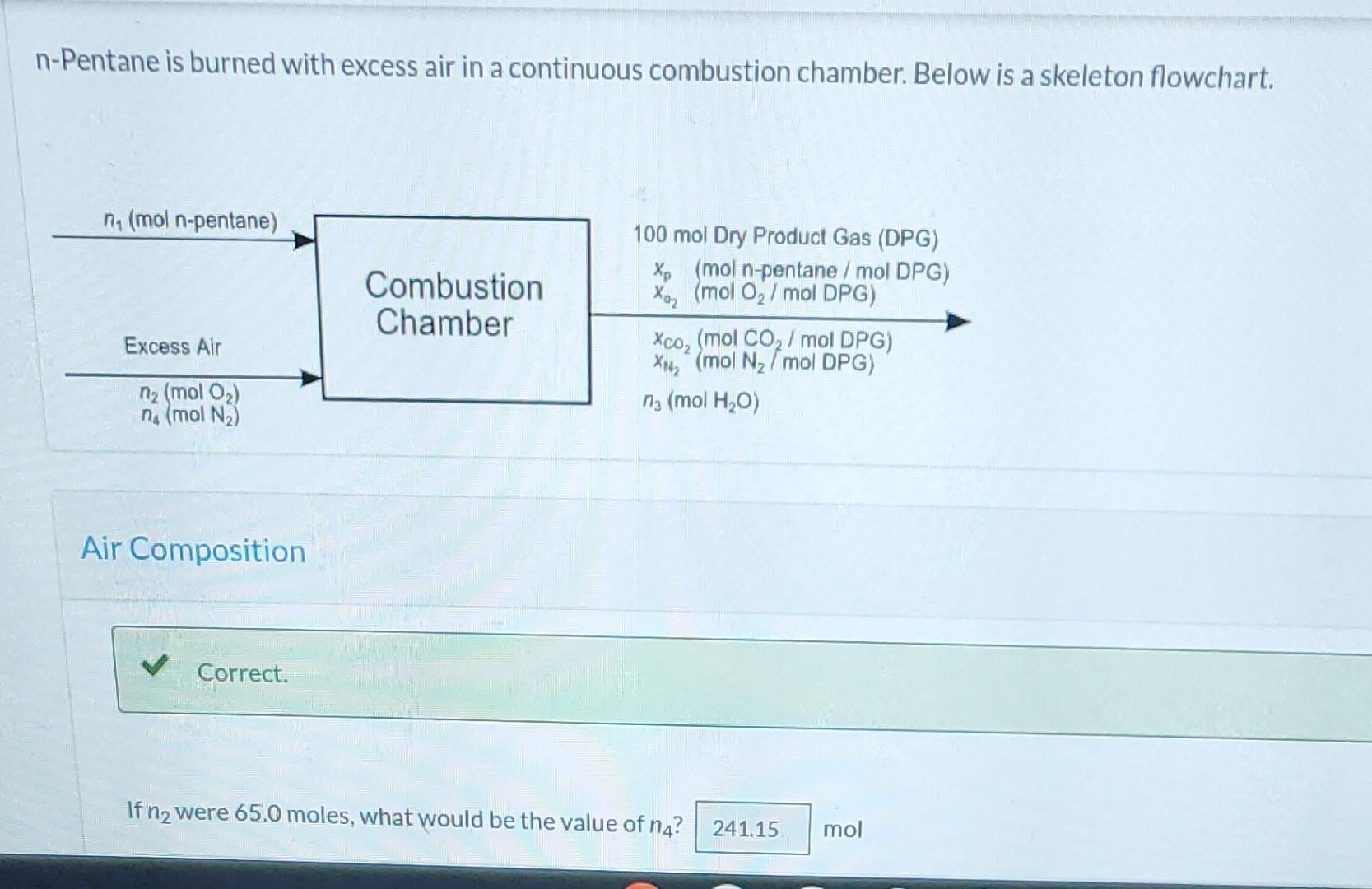
Question: Please show all work. n-Pentane is burned with excess air in a continuous combustion chamber. Below is a skeleton flowchart. n (mol n-pentane) 100 mol

Please show all work.
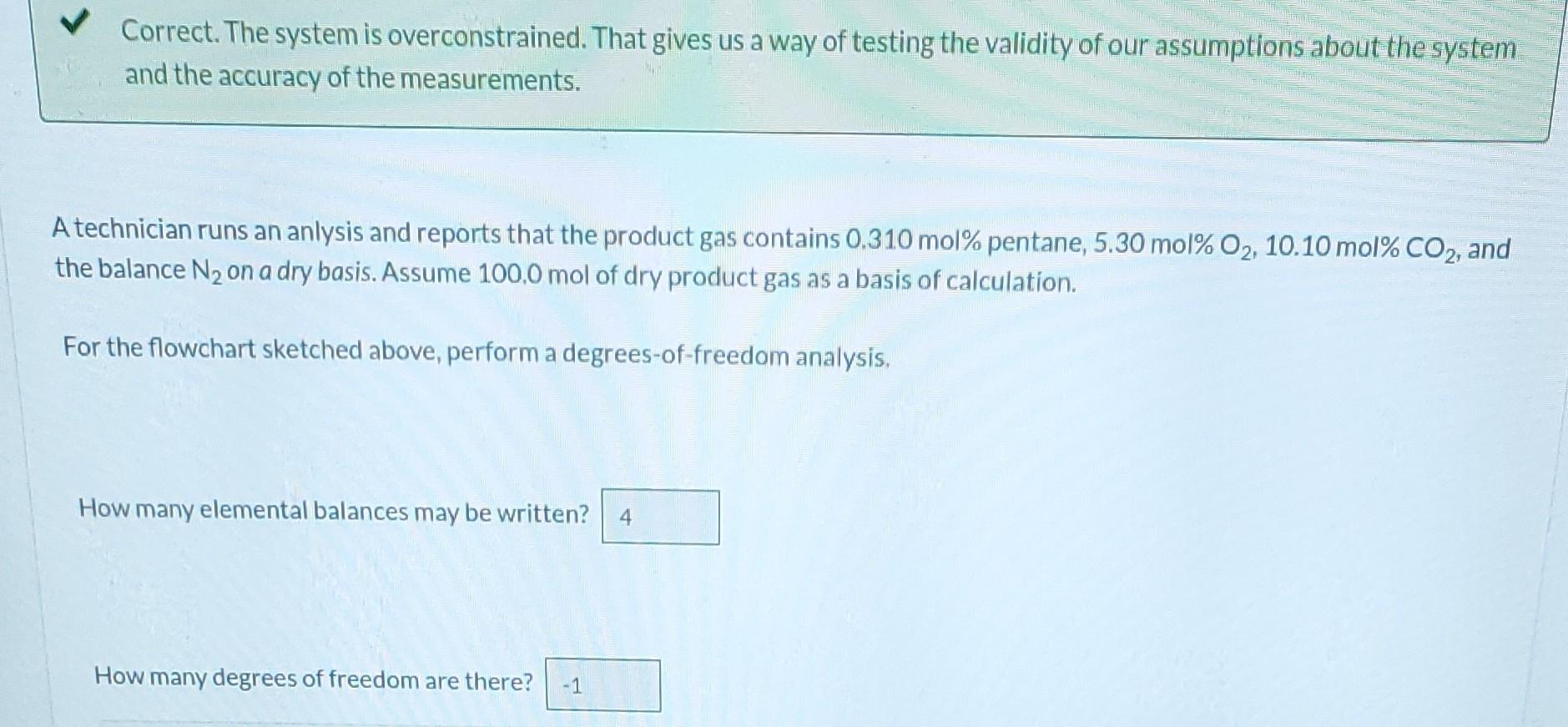
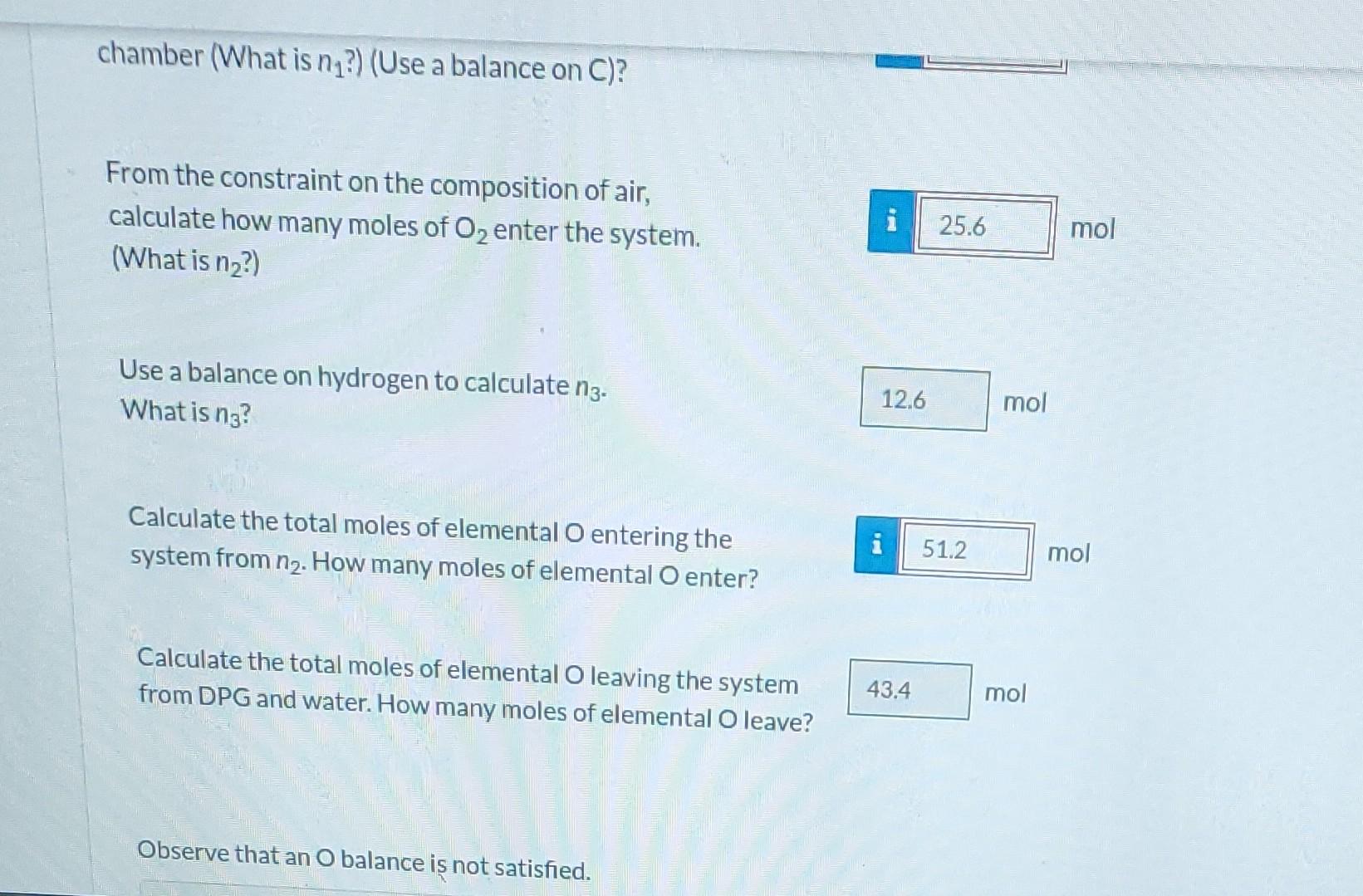
n-Pentane is burned with excess air in a continuous combustion chamber. Below is a skeleton flowchart. n (mol n-pentane) 100 mol Dry Product Gas (DPG) Xp (mol n-pentane/mol DPG) Xa (mol O/mol DPG) Combustion Chamber Excess Air XCO (mol CO/mol DPG) XN (mol N/mol DPG) n (mol O) n3 (mol HO) n (mol N) Air Composition Correct. If n were 65.0 moles, what would be the value of n4? 241.15 mol Correct. The system is overconstrained. That gives us a way of testing the validity of our assumptions about the system and the accuracy of the measurements. A technician runs an anlysis and reports that the product gas contains 0.310 mol% pentane, 5.30 mol % O2, 10.10 mol% CO2, and the balance N on a dry basis. Assume 100.0 mol of dry product gas as a basis of calculation. For the flowchart sketched above, perform a degrees-of-freedom analysis, How many elemental balances may be written? 4 How many degrees of freedom are there? -1 A technician runs an analysis and reports that the product gas contains 0.310 mol% pentane, 5.30 mol % O, 10.10 mol% CO2, and the balance N on a dry basis. Assume 100.0 mol of dry product gas as a basis of calculation. 81.5 mol N2 How many moles of N are in the dry product gas? How many moles of C are in the dry product gas? i 21.6 5.14 How many moles of n-pentane enter the combustion chamber (What is n?) (Use a balance on C)? 25.6 From the constraint on the composition of air, calculate how many moles of O enter the system. (What is n?) Use a balance on hydrogen to calculate n3. What is no? 124 i 12.6 mol mol C mol mol chamber (What is n?) (Use a balance on C)? From the constraint on the composition of air, calculate how many moles of O enter the system. (What is n?) Use a balance on hydrogen to calculate ng. What is na? Calculate the total moles of elemental O entering the system from n. How many moles of elemental O enter? Calculate the total moles of elemental O leaving the system from DPG and water. How many moles of elemental O leave? Observe that an O balance is not satisfied. A 12.6 43.4 25.6 51.2 mol mol mol mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


