Question: please show all work, thank you! Problem. 2 (15 pts) A gas within a piston-cylinder assembly undergoes and expansion from a state P1,V1 to a
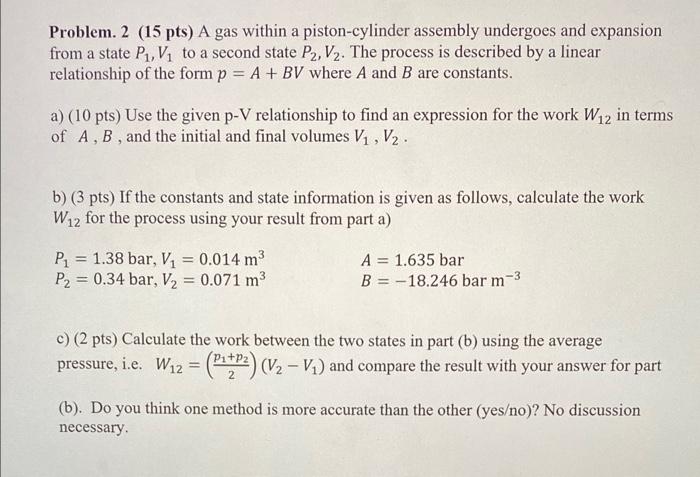
Problem. 2 (15 pts) A gas within a piston-cylinder assembly undergoes and expansion from a state P1,V1 to a second state P2,V2. The process is described by a linear relationship of the form p=A+BV where A and B are constants. a) (10 pts) Use the given p-V relationship to find an expression for the work W12 in terms of A,B, and the initial and final volumes V1,V2. b) ( 3 pts) If the constants and state information is given as follows, calculate the work W12 for the process using your result from part a) P1=1.38bar,V1=0.014m3P2=0.34bar,V2=0.071m3A=1.635barB=18.246barm c) ( 2 pts) Calculate the work between the two states in part (b) using the average pressure, i.e. W12=(2p1+p2)(V2V1) and compare the result with your answer for part (b). Do you think one method is more accurate than the other (yeso)? No discussion necessary
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


