Question: Please show all work to get to given answer Data for the volume change on mixing at 298K for methyl formate (MF)+ methanol (O) and
Please show all work to get to given answer
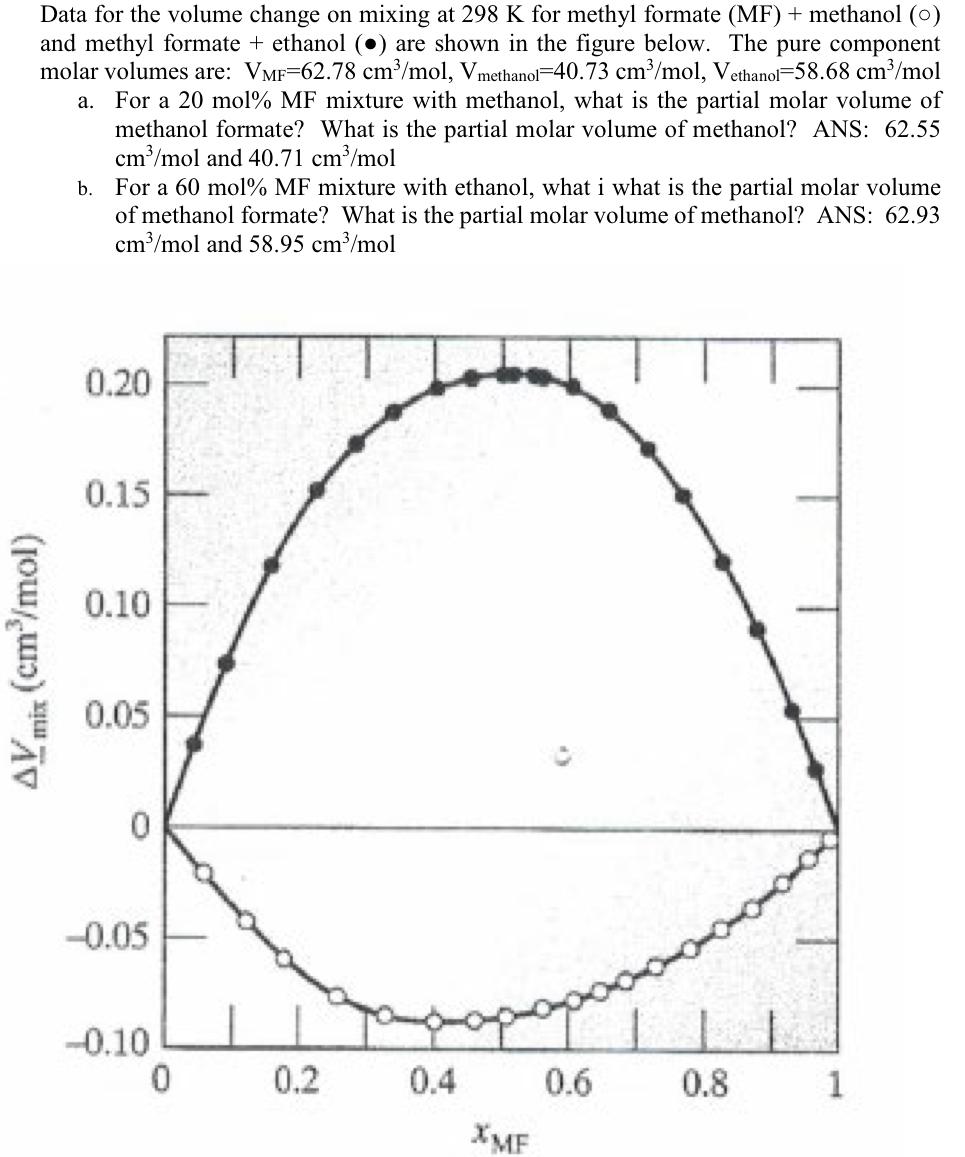
Data for the volume change on mixing at 298K for methyl formate (MF)+ methanol (O) and methyl formate + ethanol () are shown in the figure below. The pure component molar volumes are: VMF=62.78cm3/mol,Vmethanol=40.73cm3/mol,Vethanol=58.68cm3/mol a. For a 20mol%MF mixture with methanol, what is the partial molar volume of methanol formate? What is the partial molar volume of methanol? ANS: 62.55 cm3/mol and 40.71cm3/mol b. For a 60mol% MF mixture with ethanol, what i what is the partial molar volume of methanol formate? What is the partial molar volume of methanol? ANS: 62.93 cm3/mol and 58.95cm3/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


