Question: Please show all your work and write neatly: Two insulated tanks, each having a volume of 1m5 are connected to each other with an insulated
Please show all your work and write neatly:
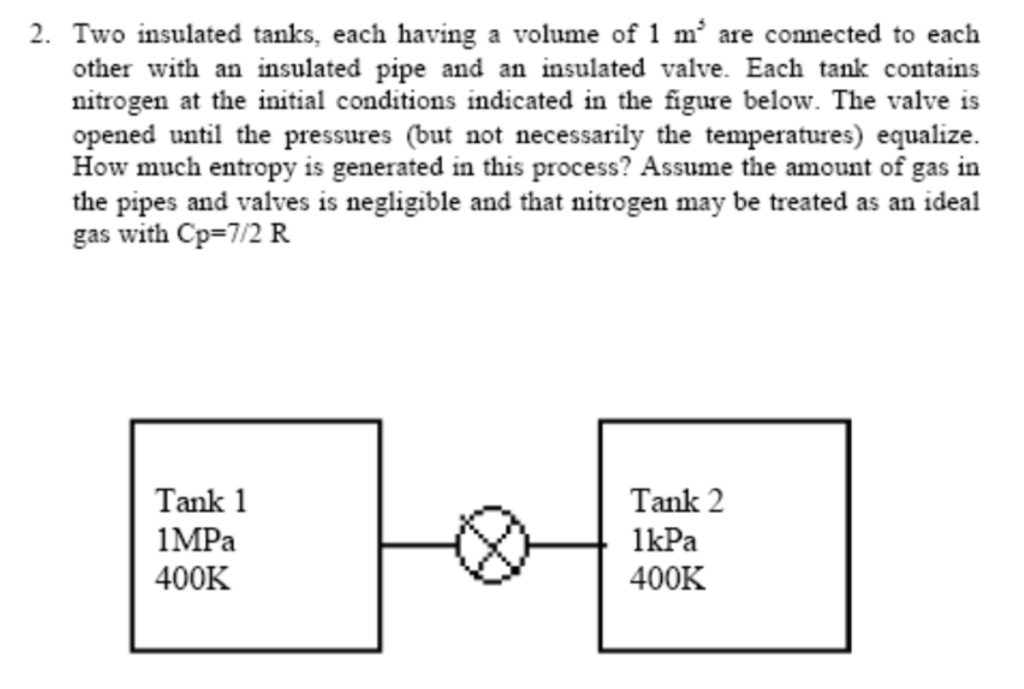
Two insulated tanks, each having a volume of 1m5 are connected to each other with an insulated pipe and an insulated valve. Each tank contains nitrogen at the initial conditions indicated in the figure below. The valve is opened until the pressures (but not necessarily the temperatures) equalize. How much entropy is generated in this process? Assume the amount of gas in the pipes and valves is negligible and that nitrogen may be treated as an ideal gas with Cp=7/2R
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


