Question: please show and explain how to do this please write on paper and clearly Determine the thermodynamic equilibrium constant K at 25C for the following
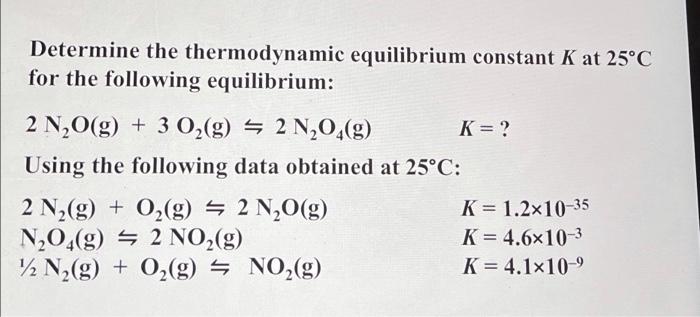
Determine the thermodynamic equilibrium constant K at 25C for the following equilibrium: 2N2O(g)+3O2(g)2N2O4(g)K=? Using the following data obtained at 25C : 2N2(g)+O2(g)2N2O(g)N2O4(g)2NO2(g)1/2N2(g)+O2(g)NO2(g)K=1.21035K=4.6103K=4.1109
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


