Question: Please show answer and explain! I will rate! Stability of Reactive Intermediates (Section 3.9) Which requires less energy to cleave the carbon-chlorine bond of 3-chloropropene

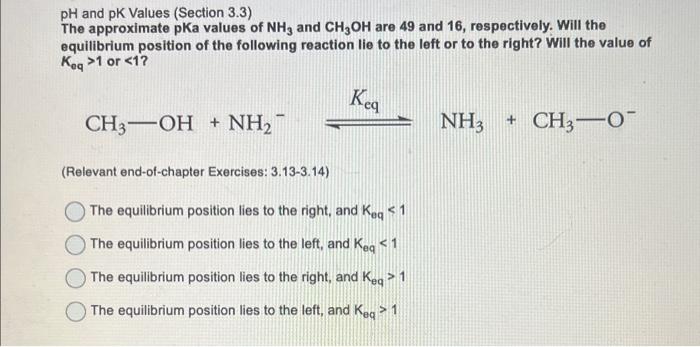
Stability of Reactive Intermediates (Section 3.9) Which requires less energy to cleave the carbon-chlorine bond of 3-chloropropene or 1 chlropropane, and why? (Relevant end-of-chapter Exercises: 3.39-3.44) 1-chloropropane, because the carbocation or carbon radical that forms afterwards is stabilized by the inductive effect from an alkyl group. 1-chloropropane, because the carbocation or carbon radical that forms afterwards is resonance-stabilized. 3-chloropropene, because the carbocation or carbon radical that forms afterwards is destabilized by resonance. 3-chloropropene, because the carbocation or carbon radical that forms afterwards is stabilized by resonance. pH and pK Values (Section 3.3) The approximate pKa values of NH3 and CH3OH are 49 and 16, respectively. Will the equilibrium position of the following reaction lie to the left or to the right? Will the value of Keq>1 or 1 The equilibrium position lies to the left, and Keq>1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


