Question: Please show clear and complete step-by-step calculations. 5-14 The dehydration butanol of alumina is carried out over a silica-alumina catalyst at 680K. CH3CH2CH2CH2OHcatCH3CH=CHCH3+H2O The rate
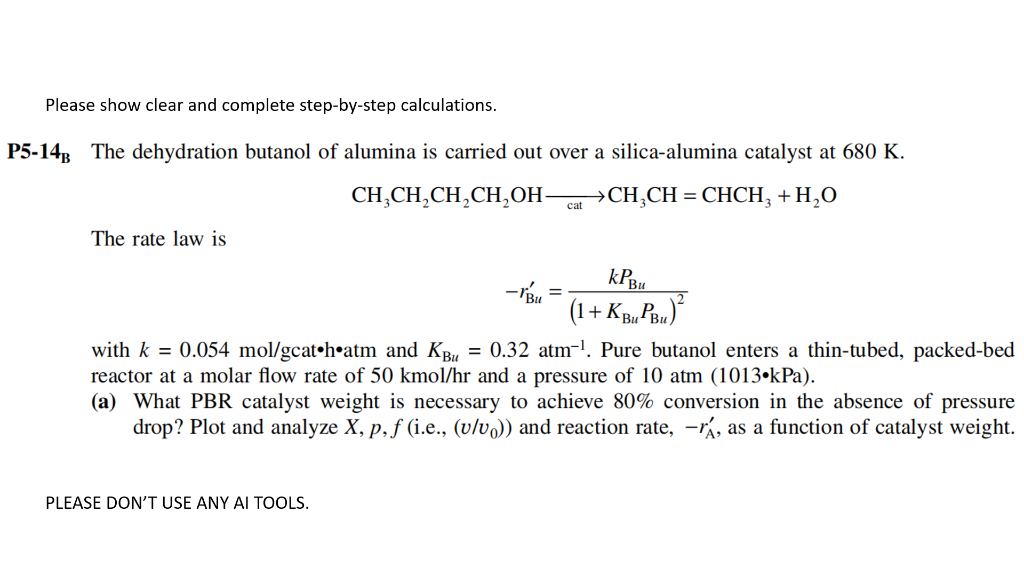
Please show clear and complete step-by-step calculations. 5-14 The dehydration butanol of alumina is carried out over a silica-alumina catalyst at 680K. CH3CH2CH2CH2OHcatCH3CH=CHCH3+H2O The rate law is rBu=(1+KBuPBu)2kPBu with k=0.054mol/gcatcotm and KBu=0.32atm1. Pure butanol enters a thin-tubed, packed-bed reactor at a molar flow rate of 50kmol/hr and a pressure of 10atm(1013kPa). (a) What PBR catalyst weight is necessary to achieve 80% conversion in the absence of pressure drop? Plot and analyze X,p,f (i.e., (v/v0)) and reaction rate, rA, as a function of catalyst weight. PLEASE DON'T USE ANY AI TOOLS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


