Question: please show clear steps = (16) The equilibrium constant for the formation of ion pairs (i.e., the association) in CuSO4 is K;= 230, in water
please show clear steps
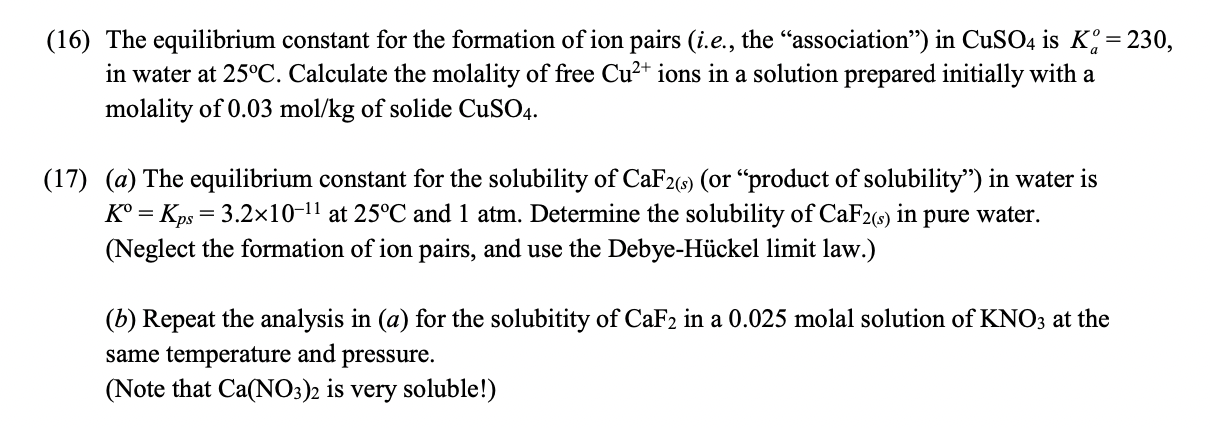
= (16) The equilibrium constant for the formation of ion pairs (i.e., the association) in CuSO4 is K;= 230, in water at 25C. Calculate the molality of free Cu2+ ions in a solution prepared initially with a molality of 0.03 mol/kg of solide CuSO4. (17) (a) The equilibrium constant for the solubility of CaF2(s) (or product of solubility'') in water is K = Kps = 3.2x10-11 at 25C and 1 atm. Determine the solubility of CaF2(s) in pure water. (Neglect the formation of ion pairs, and use the Debye-Hckel limit law.) = (b) Repeat the analysis in (a) for the solubitity of CaF2 in a 0.025 molal solution of KNO3 at the same temperature and pressure. (Note that Ca(NO3)2 is very soluble!)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


