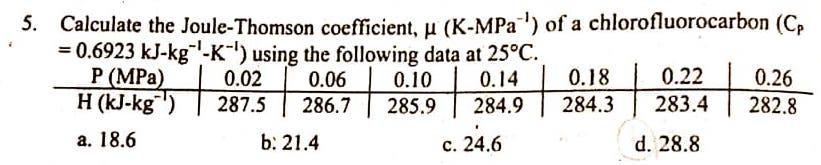
Question: PLEASE SHOW COMPLETE SOLUTION AND PROOF WHY OPTION D IS THE CORRECT ANSWER. THANK YOU! 5. Calculate the Joule-Thomson coefficient, H (K-MPa ') of a
 PLEASE SHOW COMPLETE SOLUTION AND PROOF WHY OPTION D IS THE CORRECT ANSWER. THANK YOU!
PLEASE SHOW COMPLETE SOLUTION AND PROOF WHY OPTION D IS THE CORRECT ANSWER. THANK YOU!
5. Calculate the Joule-Thomson coefficient, H (K-MPa ') of a chlorofluorocarbon (Cp = 0.6923 kJ-kg -K-') using the following data at 25C. P (MPa) 0.02 0.06 0.10 0.14 0.18 0.22 0.26 H (KJ-kg) 287.5 286.7 285.9 284.9 284.3 283.4 282.8 a. 18.6 b: 21.4 c. 24.6 d. 28.8 +
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


