Question: please show full solution ( (6) , PCCIA A liquid mixture of CCl4 et CHC13 with xCiRCI , 0.5242 at 40C has a total vapour
please show full solution
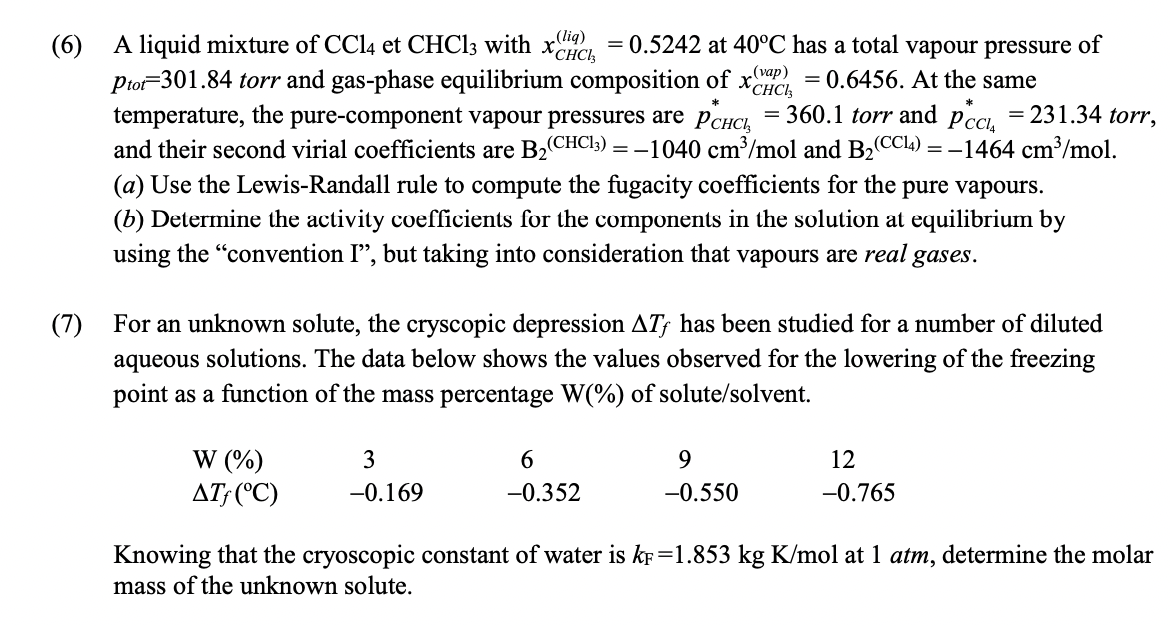
( (6) , PCCIA A liquid mixture of CCl4 et CHC13 with xCiRCI , 0.5242 at 40C has a total vapour pressure of Prof=301.84 torr and gas-phase equilibrium composition of xarc = 0.6456. At the same temperature, the pure-component vapour pressures are Pchci = 360.1 torr and = 231.34 torr, and their second virial coefficients are BZ (CHCI3) = -1040 cm3/mol and B2 (CCl4) = -1464 cm3/mol. (a) Use the Lewis-Randall rule to compute the fugacity coefficients for the pure vapours. (b) Determine the activity coefficients for the components in the solution at equilibrium by using the convention I, but taking into consideration that vapours are real gases. (7) For an unknown solute, the cryscopic depression ATf has been studied for a number of diluted aqueous solutions. The data below shows the values observed for the lowering of the freezing point as a function of the mass percentage W%) of solute/solvent. W (%) 3 -0.169 6 -0.352 9 -0.550 12 -0.765 ATF (C) Knowing that the cryoscopic constant of water is kf=1.853 kg K/ mol at 1 atm, determine the molar mass of the unknown solute
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


