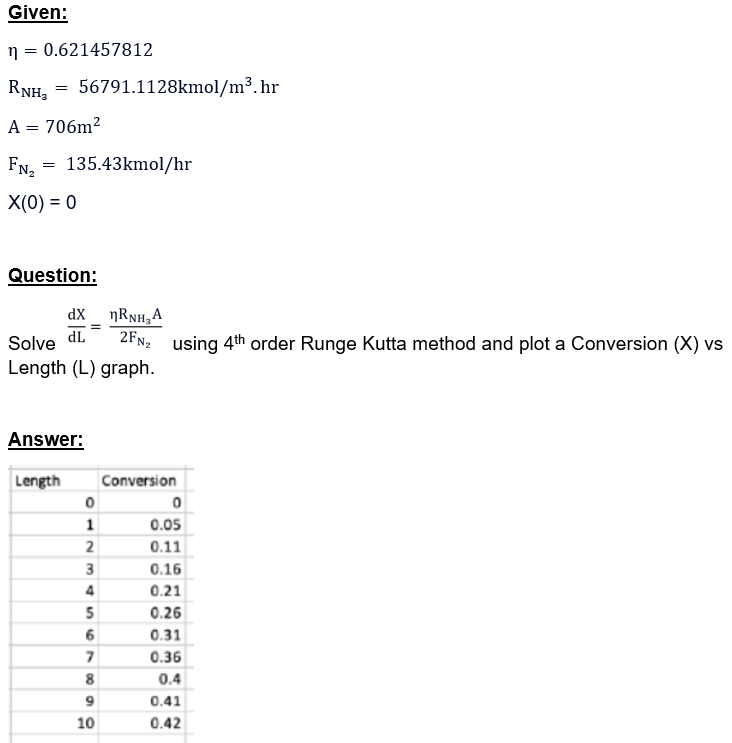
Question: Please show how to get these values in the table. Given: =0.621457812RNH3=56791.1128kmol/m3hrA=706m2FN2=135.43kmol/hrX(0)=0 Question: Solve dLdX=2FN2RNH3A using 4th order Runge Kutta method and plot a Conversion
Please show how to get these values in the table.
Given: =0.621457812RNH3=56791.1128kmol/m3hrA=706m2FN2=135.43kmol/hrX(0)=0 Question: Solve dLdX=2FN2RNH3A using 4th order Runge Kutta method and plot a Conversion (X) vs Length (L) graph
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


