Question: please , show me the steps this is 8 and 9 has table only Using the information in the table, the value of the rate
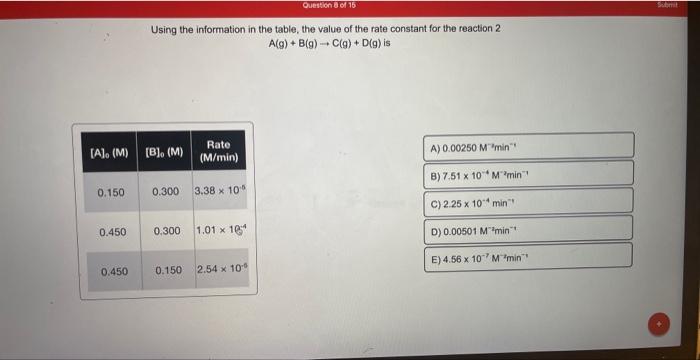
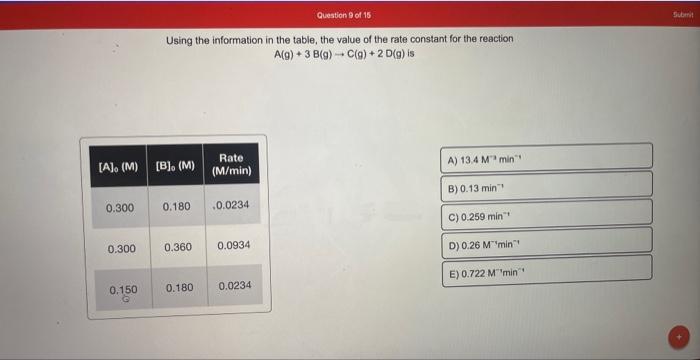
Using the information in the table, the value of the rate constant for the reaction 2A(g)+B(g)C(g)+D(g) is Using the information in the table, the value of the rate constant for the reaction A(g)+3B(g)C(g)+2D(g) is How long will it take for the concentration of A to decrease from 0.500M to 0.180M in the first-order reaction AB ? (k =0.800s1) A reaction was shown to follow second-order kinetics. How much time is required for [A] to change from 0.500M to 0.155M ? (k=0.456M1s1) What is the rate constant of a first-order reaction when 20.0% of a reactant remains after 55.5s ? A(g)+B(g)C(g)+D(g) is Using the information in the table, the value of the rate constant for the reaction A(g)+3B(g)C(g)+2D(g)is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


