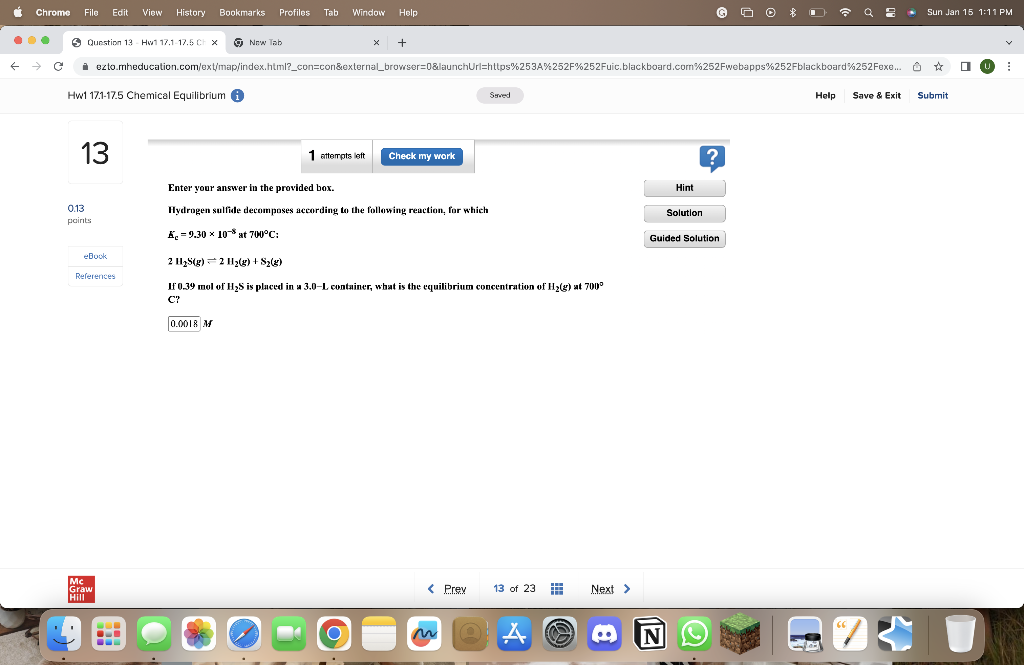
Question: Please show solution! Thanks! Enter your answer in the provided box. Hydrogen sulfide decomposes according to the following reaction, for which Kc=9.30108at700C:2H2S(g)2H2(g)+S2(g) If 0.39 mol
Please show solution! Thanks!
Enter your answer in the provided box. Hydrogen sulfide decomposes according to the following reaction, for which Kc=9.30108at700C:2H2S(g)2H2(g)+S2(g) If 0.39 mol of H2S is pluced in a 3.0L container, what is the equilibrium concentration of H2(g) at 700 C? M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


