Question: Please show step by step how the equations were derived. Based on the 1st order kinetic and Langmuir adsorption isotherm to derive the rate equation
Please show step by step how the equations were derived.
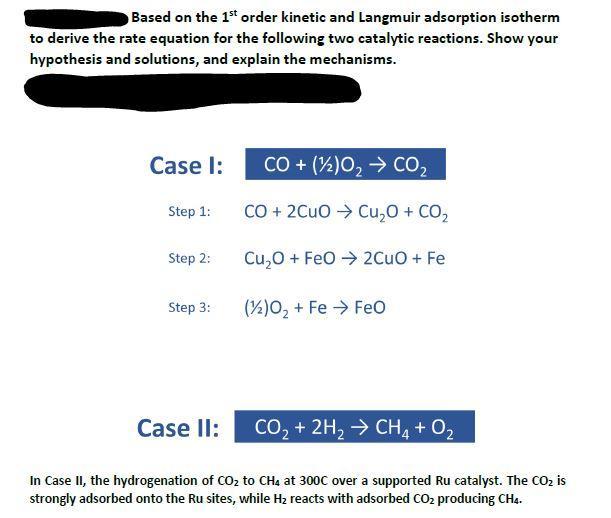
Based on the 1st order kinetic and Langmuir adsorption isotherm to derive the rate equation for the following two catalytic reactions. Show your hypothesis and solutions, and explain the mechanisms. Case I: Step 1: CO+2CuOCu2O+CO2 Step 2: Cu2O+FeO2CuO+Fe Step 3: In Case II, the hydrogenation of CO2 to CH4 at 300C over a supported Ru catalyst. The CO2 is strongly adsorbed onto the Ru sites, while H2 reacts with adsorbed CO2 producing CH4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


