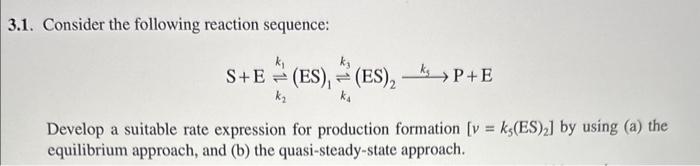
Question: please show steps to help me understand this because im confused 3.1. Consider the following reaction sequence: S+Ek2k1(ES)1k4k3(ES)2k3P+E Develop a suitable rate expression for production
 please show steps to help me understand this because im confused
please show steps to help me understand this because im confused3.1. Consider the following reaction sequence: S+Ek2k1(ES)1k4k3(ES)2k3P+E Develop a suitable rate expression for production formation [v=k5(ES)2] by using (a) the equilibrium approach, and (b) the quasi-steady-state approach
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


