Question: PLEASE SHOW THE EQUATIONS USED 9.1-18. Multicomponent Diffusion. At a total pressure of 202.6kPa and 358K, ammonia gas (A) is diffusing at steady state through
PLEASE SHOW THE EQUATIONS USED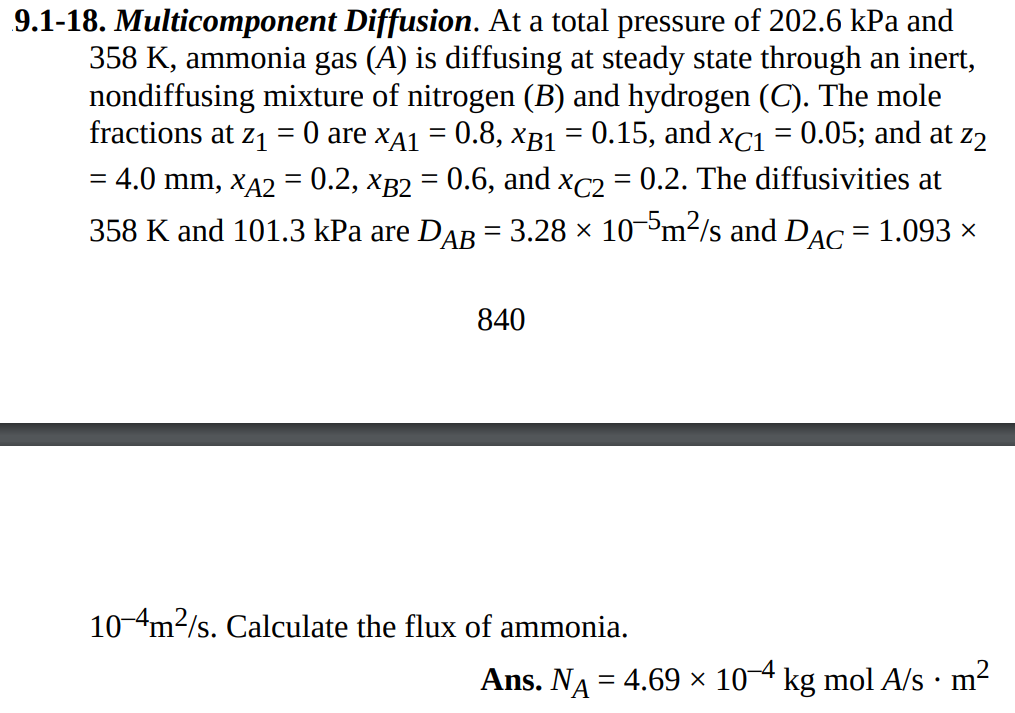
9.1-18. Multicomponent Diffusion. At a total pressure of 202.6kPa and 358K, ammonia gas (A) is diffusing at steady state through an inert, nondiffusing mixture of nitrogen (B) and hydrogen (C). The mole fractions at z1=0 are xA1=0.8,xB1=0.15, and xC1=0.05; and at z2 =4.0mm,xA2=0.2,xB2=0.6, and xC2=0.2. The diffusivities at 358K and 101.3kPa are DAB=3.28105m2/s and DAC=1.093 840 104m2/s. Calculate the flux of ammonia. Ans. NA=4.69104kgmolA/sm2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


