Question: please show work and explain the visible light question please lol Consider the Bohr model of the atom. Which transition would correspond to the highest

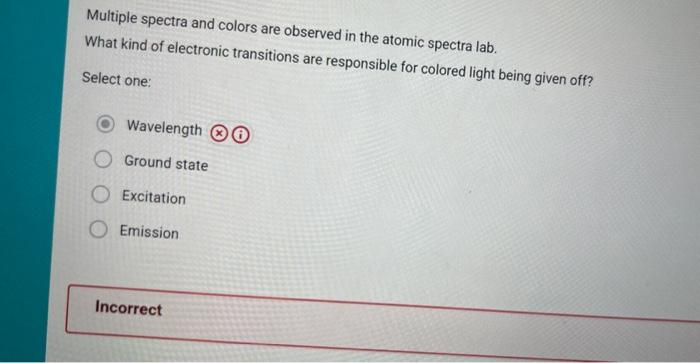

Consider the Bohr model of the atom. Which transition would correspond to the highest frequency of light emitted? Select one: n=6ton=3 n=1 to n=5 () n=4 to n=1 n=2 to n=6 n=6 to n=10 Multiple spectra and colors are observed in the atomic spectra lab. What kind of electronic transitions are responsible for colored light being given off? Select one: Wavelength Ground state Excitation Emission Visible light falls into wavelength ranges of 400700nm, for which 1m=1109mm. The energy and wavelength of light are related by the equation E=Ahr where E is energy in Joules, h is Planck's constant (6.626104J5), is the speed of light (2.998108m/s), and d is the wavelength in in. If a visible ight photon has a wavelength of 632.3nm, what is the energy of the photon (in d) ? Type
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


