Question: Please show work. i have provided the answers for this problem 5. For the reaction +RC the following data were mollected a) What is the
Please show work. i have provided the answers for this problem 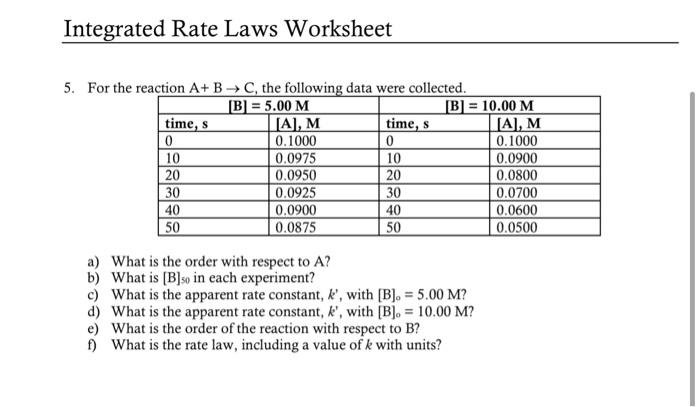
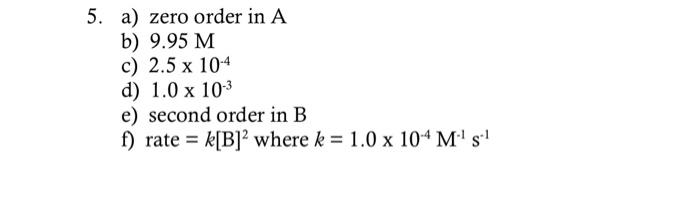

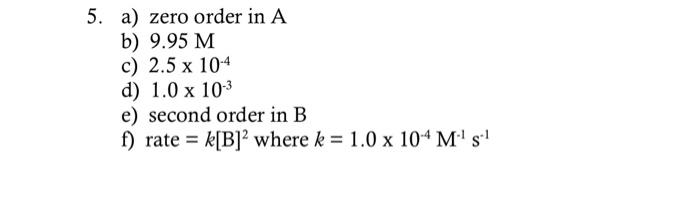
5. For the reaction +RC the following data were mollected a) What is the order with respect to A ? b) What is [B]50 in each experiment? c) What is the apparent rate constant, k, with [B]0=5.00M ? d) What is the apparent rate constant, k, with [B]0=10.00M ? e) What is the order of the reaction with respect to B ? f) What is the rate law, including a value of k with units? 5. a) zero order in A b) 9.95M c) 2.5104 d) 1.0103 e) second order in B f) rate =k[B]2 where k=1.0104M1s1
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


