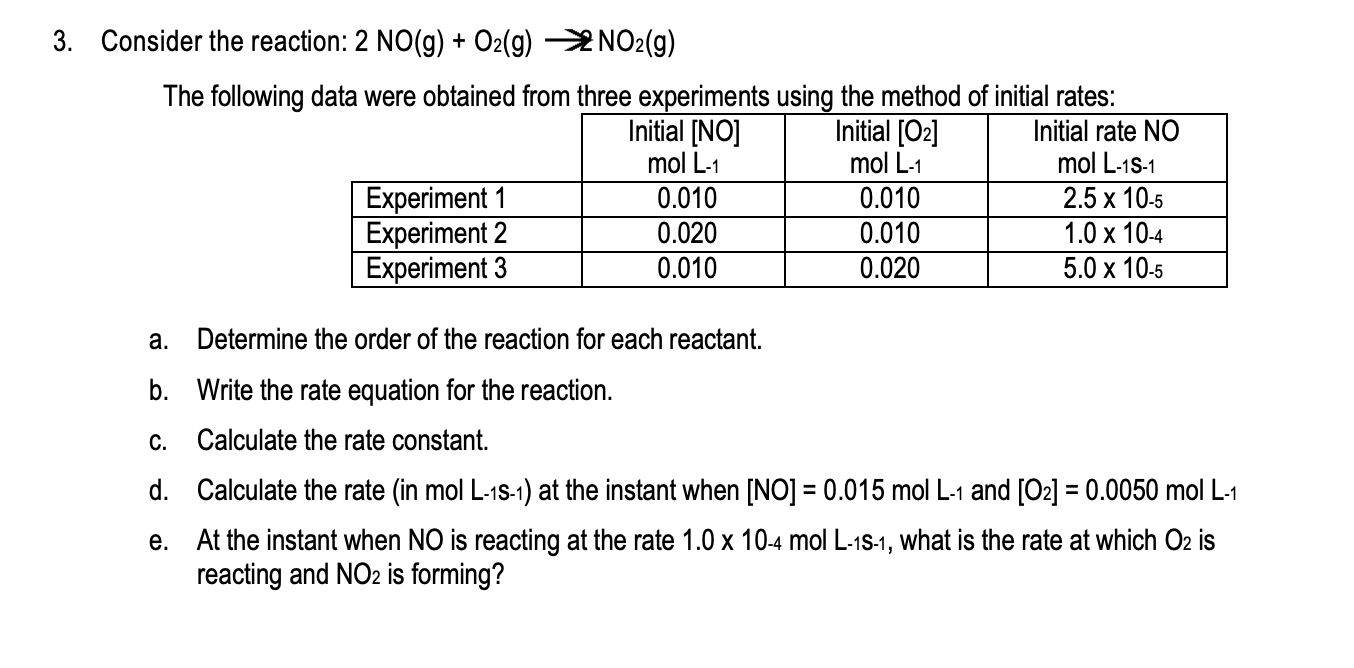
Question: please show work in as much detail as possible! 3. Consider the reaction: 2 NO(g) + O2(g) NO2(g) The following data were obtained from three
 please show work in as much detail as possible!
please show work in as much detail as possible!
3. Consider the reaction: 2 NO(g) + O2(g) NO2(g) The following data were obtained from three experiments using the method of initial rates: Initial [NO] Initial [O2] Initial rate NO mol L-1 mol L-1 mol L-15-1 Experiment 1 0.010 0.010 2.5 x 10-5 Experiment 2 0.020 0.010 1.0 x 10-4 Experiment 3 0.010 0.020 5.0 x 10-5 a. Determine the order of the reaction for each reactant. b. Write the rate equation for the reaction. C. Calculate the rate constant. d. Calculate the rate (in mol L-15-1) at the instant when [NO] = 0.015 mol L-1 and [O2] = 0.0050 mol L-1 e. At the instant when NO is reacting at the rate 1.0 x 10-4 mol L-1S-1, what is the rate at which O2 is reacting and NO2 is forming
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


