Question: Please show your work. Planck Function from Notes: Blackbody Radiation/Planck Function As a function of frequency and not wavelength 2h13 1 B(v, T) C2 hv
Please show your work.
Planck Function from Notes:
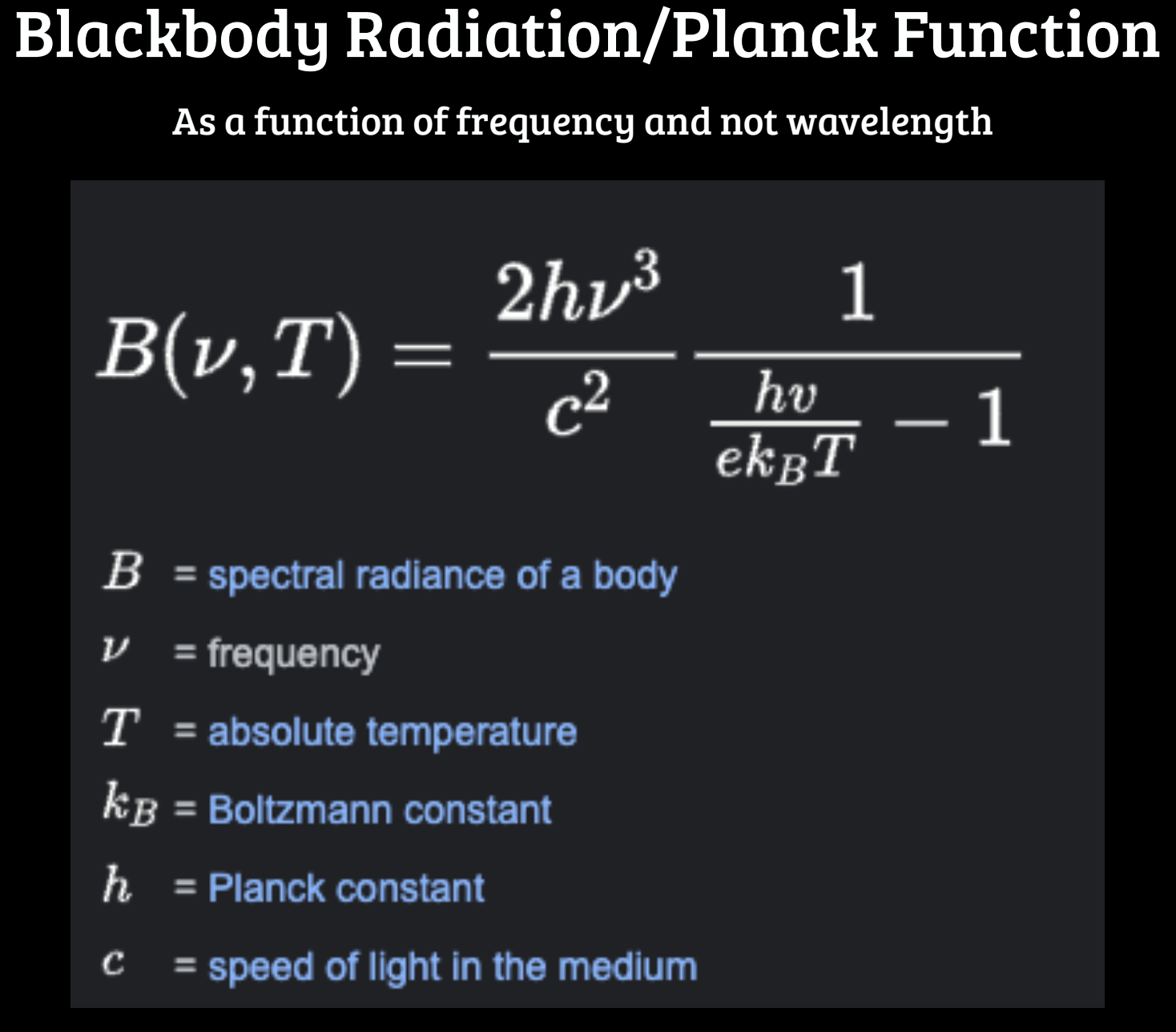

Blackbody Radiation/Planck Function As a function of frequency and not wavelength 2h13 1 B(v, T) C2 hv ekBT - 1 B = spectral radiance of a body v = frequency T = absolute temperature KB = Boltzmann constant h = Planck constant C = speed of light in the mediumLets consider the Planck Function (equation can be found in the notes): A. Starting from the Planck Function for blackbody radiation, derive the Stefan- Boltzmann law such that (5pts): T Budv = oT4 B. Starting from the Planck Function for blackbody radiation, derive Wien's law such that (5pts). : Amax T = 0.29 cm K C. A person's typical body temperature is 98.6 F and typical surface area of skin is ~1.4 m2. Assuming that the average person absorbs and emits radiation similar to a blackbody, compute the energy per second (in erg/s) radiated by an average person? (5pts) D. Compute the peak wavelength (in Angstrom) of black body radiation emitted by the average person based on the information in part (c). What part of the spectrum is this peak wavelength in? (5pt) E. On a chilly day in Austin (during the month of January), the lows are typically around 41 F. Compute the energy per second (in ergs/s) absorbed by the average person standing outside in Austin at this temperature (5pts) F. What is the net energy difference between (c) and (e) ? How does this compare to a 100W light bulb ? (5pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


