Question: Please solve all parts step by step. I ll upvote your efforts 5.2 .6 HYDROL - Batch Reactor Hydrolysis of Acetic Anhydride System The liquid

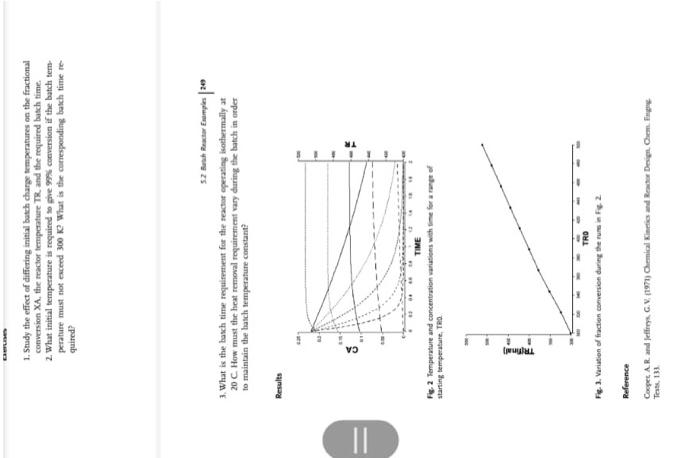
5.2 .6 HYDROL - Batch Reactor Hydrolysis of Acetic Anhydride System The liquid phase hydrolysis reaction of acetic anhydride to form acetic acid is carried out in a constant volume, adiabatic batch reactoc. The reaction is exothermic with the following stoichionetry (CH1CO)2O+H2O2CH3COOH+Heat Fig. 1. The adiabutic butch reactor with wariables of concentration and temperature. Model The acetic anhydride balance can be formulated as (Rateofaccumulationinreactor)=(Rateoflossduetochemicalreaction) hence, for the first-order reaction dtd(vCA)=kCsv and for constant volume conditions dtdCA=kCCA 243 5 simulation fook and Eromples of Cheminal Engivering Prorent The fractional couversion for the batch is xk=CaiCAaCA The temperature influence on the rate is given by k=Ze1/kTR The beat balance under adubatic conditions is as follow: 1. Study the effect of differing initial butch charge trmperatures on the ftactional conversion XA, the reactor temperature TR, and the required basch time. 2. What initial temperature is requited to phe 9 ghi conversion if the batch temperature must not eaceed 300K. What is the carresponding bakch time te quired? 5.2 Buak Reator Enamplin | 243 3. What is the bash time requifmnent for the seactor operating isochermally at 20C How must the heat removal roquirement vary daring the batch in arder to maintain the basch temperature constant? Results Fig. 2 lempeature and concentration warations whth time for a rager of starting temperatute, the Fig. 1. viriation of fraction comention during the turs in Fe. 2 Reference Teasc, 133 5.2 .6 HYDROL - Batch Reactor Hydrolysis of Acetic Anhydride System The liquid phase hydrolysis reaction of acetic anhydride to form acetic acid is carried out in a constant volume, adiabatic batch reactoc. The reaction is exothermic with the following stoichionetry (CH1CO)2O+H2O2CH3COOH+Heat Fig. 1. The adiabutic butch reactor with wariables of concentration and temperature. Model The acetic anhydride balance can be formulated as (Rateofaccumulationinreactor)=(Rateoflossduetochemicalreaction) hence, for the first-order reaction dtd(vCA)=kCsv and for constant volume conditions dtdCA=kCCA 243 5 simulation fook and Eromples of Cheminal Engivering Prorent The fractional couversion for the batch is xk=CaiCAaCA The temperature influence on the rate is given by k=Ze1/kTR The beat balance under adubatic conditions is as follow: 1. Study the effect of differing initial butch charge trmperatures on the ftactional conversion XA, the reactor temperature TR, and the required basch time. 2. What initial temperature is requited to phe 9 ghi conversion if the batch temperature must not eaceed 300K. What is the carresponding bakch time te quired? 5.2 Buak Reator Enamplin | 243 3. What is the bash time requifmnent for the seactor operating isochermally at 20C How must the heat removal roquirement vary daring the batch in arder to maintain the basch temperature constant? Results Fig. 2 lempeature and concentration warations whth time for a rager of starting temperatute, the Fig. 1. viriation of fraction comention during the turs in Fe. 2 Reference Teasc, 133
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


