Question: Please solve all the parts QUESTION 1 (25 marks) a) i. Explain the concept of water as both, proton donor and acceptor in Acid-Base equilibrium
 Please solve all the parts
Please solve all the parts
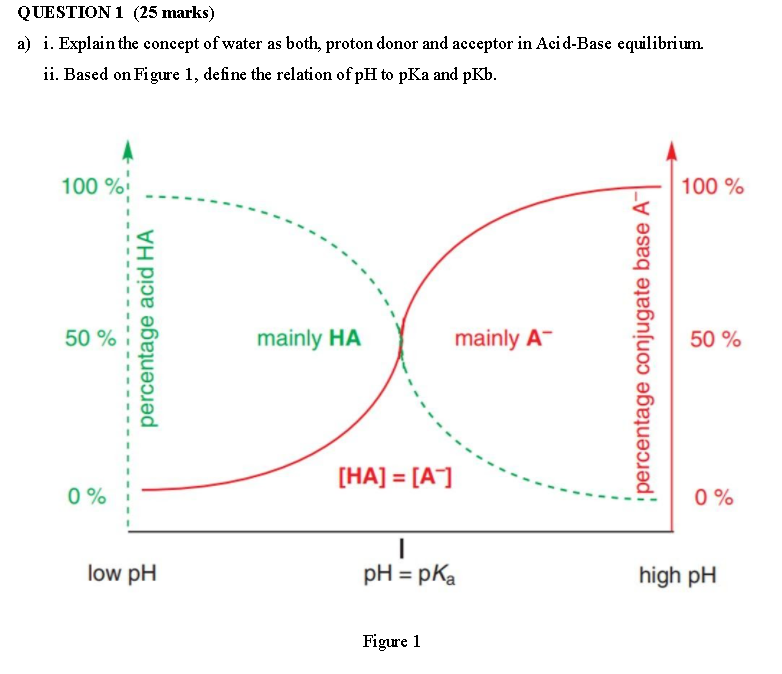
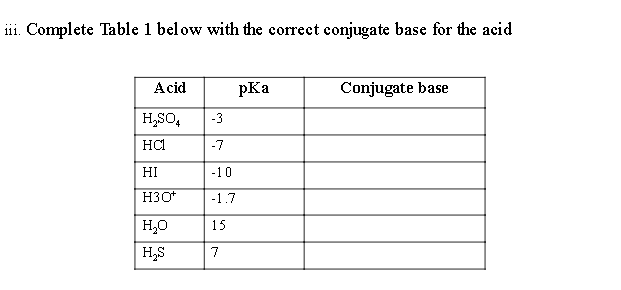
QUESTION 1 (25 marks) a) i. Explain the concept of water as both, proton donor and acceptor in Acid-Base equilibrium ii. Based on Figure 1, define the relation of pH to pKa and pKb. 100 % 100 % 50 % percentage acid HA mainly HA mainly A- percentage conjugate base A- 50 % [HA] = [A] 0% 0% low pH pH = pka high pH Figure 1 iii. Complete Table 1 below with the correct conjugate base for the acid Acid Conjugate base H,SO -3 HCI -7 HI -10 H3O+ -1.7 15 HO HS 7
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


