Question: please solve and show all steps and calculation Consider the following reaction: N2(g)+3H2(g)2NH3(g) In a given experiment, 1.63 moles of N2(g) and 3.50 moles of
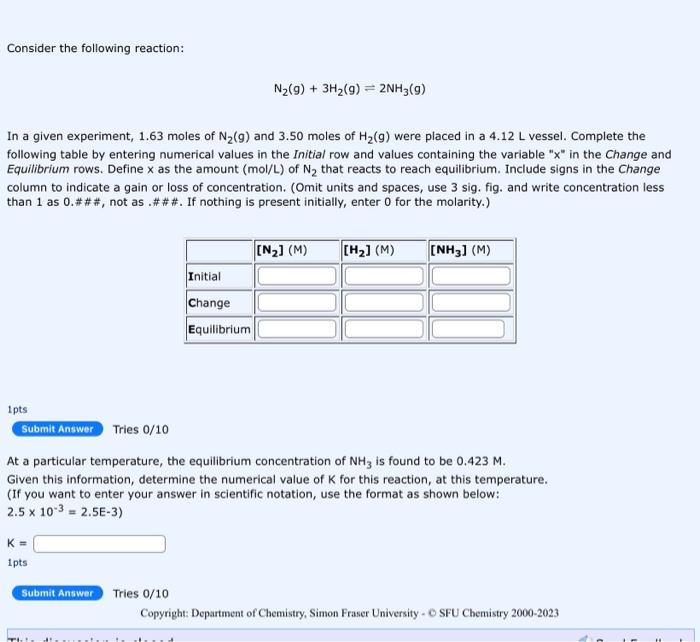
Consider the following reaction: N2(g)+3H2(g)2NH3(g) In a given experiment, 1.63 moles of N2(g) and 3.50 moles of H2(g) were placed in a 4.12L vessel. Complete the following table by entering numerical values in the Initial row and values containing the variable " x " in the Change and Equilibrium rows. Define x as the amount (mol/L ) of N2 that reacts to reach equilibrium. Include signs in the Change column to indicate a gain or loss of concentration. (Omit units and spaces, use 3 sig. fig. and write concentration less than 1 as 0.###, not as.\#\#\#. If nothing is present initially, enter 0 for the molarity.) 1pts Tries 0/10 At a particular temperature, the equilibrium concentration of NH3 is found to be 0.423M. Given this information, determine the numerical value of K for this reaction, at this temperature. (If you want to enter your answer in scientific notation, use the format as shown below: 2.5103=2.5E3) K= 1pts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


