Question: please solve and show all steps and calculation Le Chatelier's Principle II Is the statement true or false, with respect to the specified reaction in
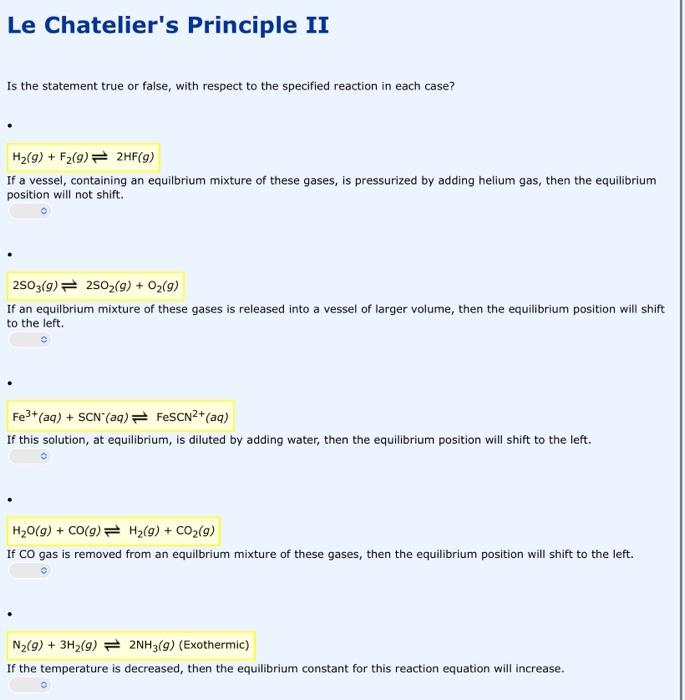
Le Chatelier's Principle II Is the statement true or false, with respect to the specified reaction in each case? If a vessel, containing an equilbrium mixture of these gases, is pressurized by adding helium gas, then the equilibrium position will not shift. 2SO3(g)2SO2(g)+O2(g) If an equilbrium mixture of these gases is released into a vessel of larger volume, then the equilibrium position will shift to the left. Fe3+(aq)+SCN(aq)FeSCN2+(aq) If this solution, at equilibrium, is diluted by adding water, then the equilibrium position will shift to the left. H2O(g)+CO(g)H2(g)+CO2(g) If CO gas is removed from an equilbrium mixture of these gases, then the equilibrium position will shift to the left. N2(g)+3H2(g)2NH3(g)(Exothermic) If the temperature is decreased, then the equilibrium constant for this reaction equation will increase
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


