Question: please solve ASAP (13 points) How much energy is required for 350.0g of carbon tetrachloride, CCl4(MW= 153.8g/mol ), to go from 20.0C to 95.0C ?
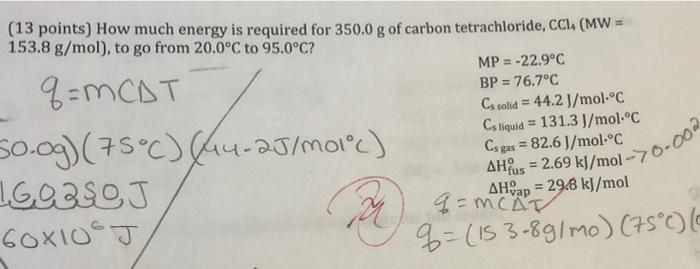
(13 points) How much energy is required for 350.0g of carbon tetrachloride, CCl4(MW= 153.8g/mol ), to go from 20.0C to 95.0C ? q=mCDTMP=22.9CBP=76.7CCssolid=44.2J/molCCsliquld=131.3J/molCCsgas=82.6J/molCHfus=2.69kJ/mol70.0021,6,0350,Jq0=m(Hvapo=29.8kJ/mol60106Ja=(153.8g/mo)(75C)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


