Question: please solve asap with clear steps thanks in advance QUESTION 1 (25 marks) A student conducted a crystallization experiment and estimated that the total heat
please solve asap with clear steps thanks in advance
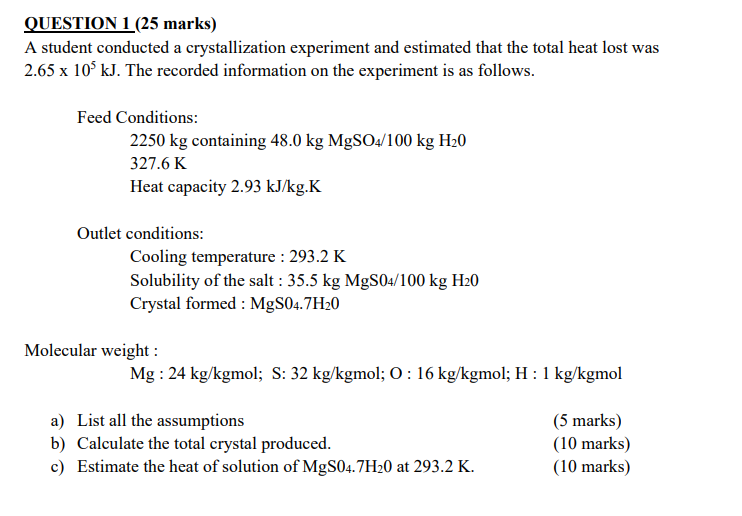
QUESTION 1 (25 marks) A student conducted a crystallization experiment and estimated that the total heat lost was 2.65105kJ. The recorded information on the experiment is as follows. Feed Conditions: 2250kgcontaining48.0kgMgSO/100kgH20327.6KHeatcapacity2.93kJ/kg.K Heat capacity 2.93kJ/kgK Outlet conditions: Cooling temperature : 293.2K Solubility of the salt : 35.5kgMgS04/100kgH20 Crystal formed : MgS4.7H2O Molecular weight : Mg:24kg/kgmol;S:32kg/kgmol;O:16kg/kgmol;H:1kg/kgmol a) List all the assumptions b) Calculate the total crystal produced. c) Estimate the heat of solution of MgS47H20 at 293.2K. (5 marks) (10 marks) (10 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


