Question: please solve:) At 397K, this reaction has a Kc value of 0.0663. 2X(g)+2Y(g)Z(g) Calculate Kp at 397K. Note that the pressure is in units of
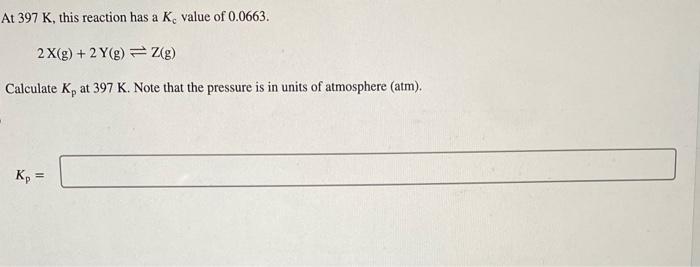
At 397K, this reaction has a Kc value of 0.0663. 2X(g)+2Y(g)Z(g) Calculate Kp at 397K. Note that the pressure is in units of atmosphere (atm). Kp
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


