Question: please solve by showing steps You had been given a new penny to test if it is made up of pure copper or not. You

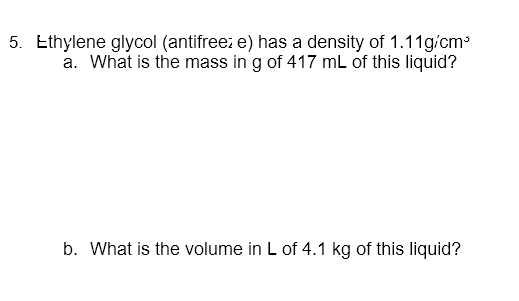 please solve by showing steps
please solve by showing steps
You had been given a new penny to test if it is made up of pure copper or not. You measured the mass of the penn y which was 2..9g. You then tind that the penny displaces 1.34.cm3 of water. Is the penniy made of pure copper? ( Density of pure copper =8.96g/cm3 ) 5. Ethylene glycol (antifreei e) has a density of 1.11gcm5 a. What is the mass in g of 417mL of this liquid? b. What is the volume in L of 4.1kg of this liquid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


