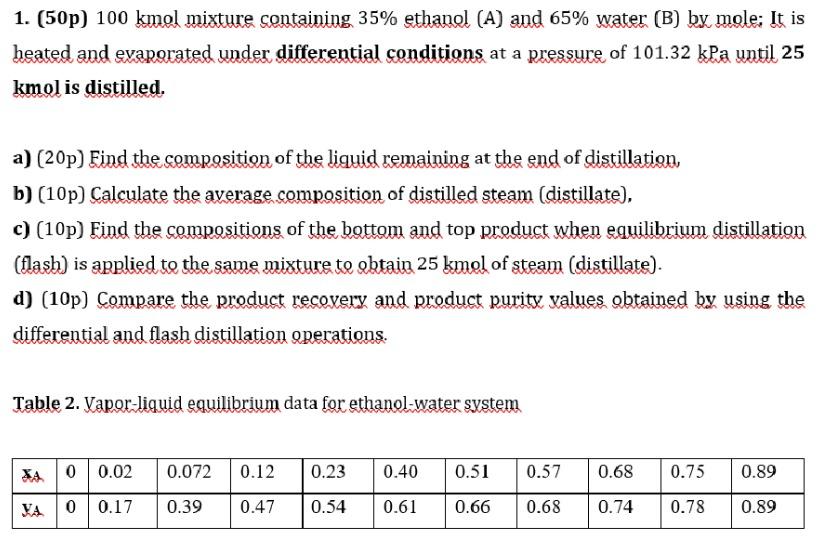
Question: Please solve fast and correct. 1. (50p) 100kmol mixture containing 35% ethanol (A) and 65% water (B) by mole; It is heated and evaporated under
 Please solve fast and correct.
Please solve fast and correct.
1. (50p) 100kmol mixture containing 35% ethanol (A) and 65% water (B) by mole; It is heated and evaporated under differential conditions at a pressure of 101.32kPa until 25 kmol is distilled. a) (20p) Find the composition of the liquid remaining at the end of distillation, b) (10p) Calculate the average composition of distilled steam (distillate), c) (10p) Find the compositions of the bottom and top product when equilibrium distillation (flash) is applied to the same mixture to obtain 25kmol of steam (distillate). d) (10p) Compare the product recovery and product purity values obtained by using the differential and flash distillation operations. Table 2. Vapor-liquid equilibrium data for ethanol-water system
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


