Question: Please solve given for the electrode, E= -412.9 mV A fluoride (ISE) has been calibrated with solutions of KF in a constant ionic strength medium
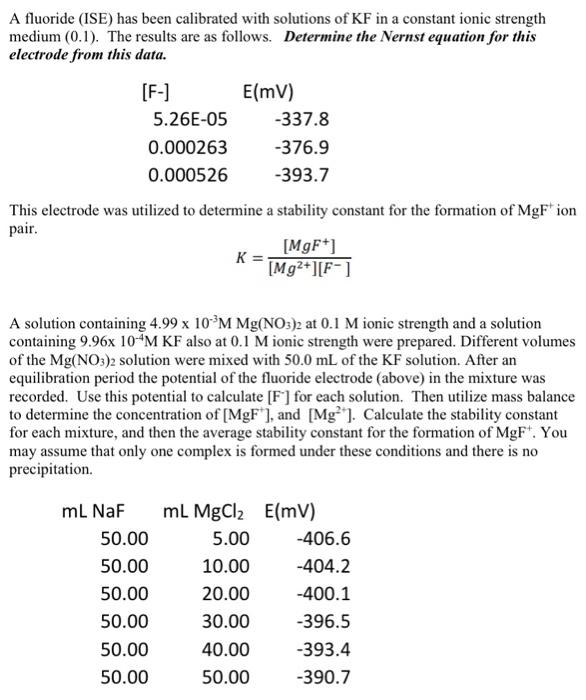
A fluoride (ISE) has been calibrated with solutions of KF in a constant ionic strength medium (0.1). The results are as follows. Determine the Nernst equation for this electrode from this data. This electrode was utilized to determine a stability constant for the formation of MgF+ion pair. K=[Mg2+][F][MgF+] A solution containing 4.99103MMg(NO3)2 at 0.1M ionic strength and a solution containing 9.96104MKF also at 0.1M ionic strength were prepared. Different volumes of the Mg(NO3)2 solution were mixed with 50.0mL of the KF solution. After an equilibration period the potential of the fluoride electrode (above) in the mixture was recorded. Use this potential to calculate [F]for each solution. Then utilize mass balance to determine the concentration of [MgF+], and [Mg2+]. Calculate the stability constant for each mixture, and then the average stability constant for the formation of MgF+. You may assume that only one complex is formed under these conditions and there is no precipitation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


