Question: please solve how much solid buffer needed for each of the solid buffers Given the following: SODIUM ACETATE: 136.08 g/mol TRIS BASE: 121.14 g/mo) HEPES:
Given the following:
SODIUM ACETATE: 136.08 g/mol
TRIS BASE: 121.14 g/mo)
HEPES: 238.03g/mol
SODIUM CITRATE: 294.1 g/mol
POTASSIUM PHOSPHATE (Monobasic): 136.08 g/mol
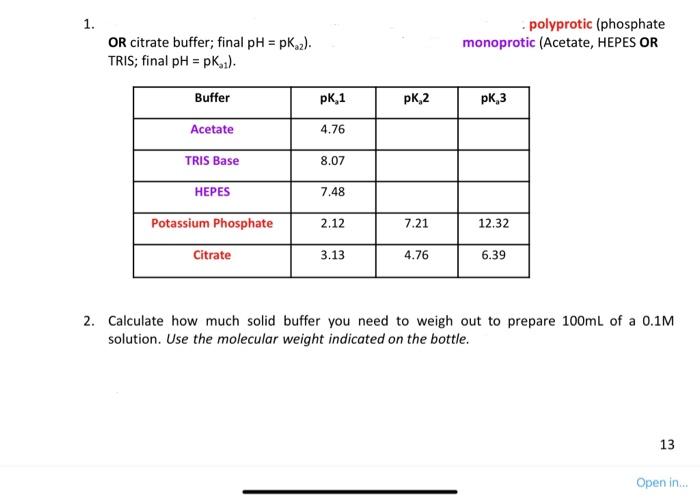
1. - polyprotic (phosphate OR citrate buffer; final pH=pKa2 ). monoprotic (Acetate, HEPES OR TRIS; final pH=pKo1). 2. Calculate how much solid buffer you need to weigh out to prepare 100mL of a 0.1M solution. Use the molecular weight indicated on the bottle
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


