Question: PLEASE SOLVE IN EXCEL USING TABLES AND SOLVER FUNCTION DO NOT SOLVE IT ON PAPER OTHERWISE YOU'LL GET REPORTED (ONLY THE EQUATIONS ON PAPER) The
PLEASE SOLVE IN EXCEL USING TABLES AND SOLVER FUNCTION DO NOT SOLVE IT ON PAPER OTHERWISE YOU'LL GET REPORTED (ONLY THE EQUATIONS ON PAPER)
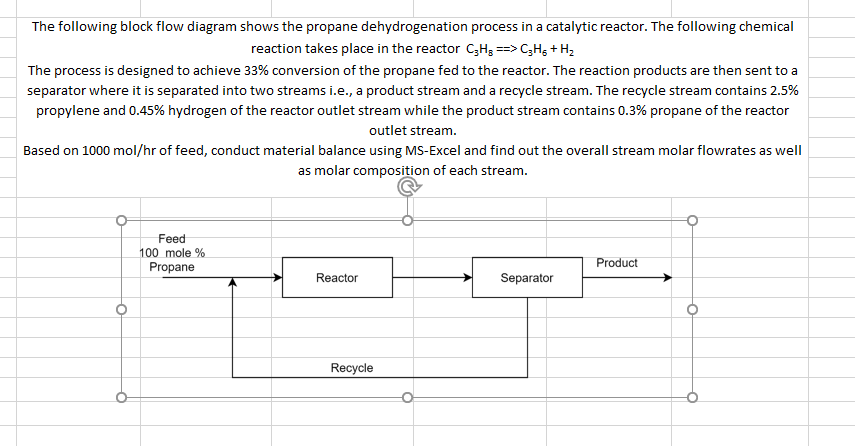
The following block flow diagram shows the propane dehydrogenation process in a catalytic reactor. The following chemical reaction takes place in the reactor C3H3 ==> C3H + H2 The process is designed to achieve 33% conversion of the propane fed to the reactor. The reaction products are then sent to a separator where it is separated into two streams i.e., a product stream and a recycle stream. The recycle stream contains 2.5% propylene and 0.45% hydrogen of the reactor outlet stream while the product stream contains 0.3% propane of the reactor outlet stream. Based on 1000 mol/hr of feed, conduct material balance using MS-Excel and find out the overall stream molar flowrates as well as molar composition of each stream. Feed 100 mole % Propane Product Reactor Separator o O Recycle
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


