Question: PLEASE SOLVE IT CORRECTLY. DO NOT COPY. The A+BL reaction is carried out in the solution phase in two series-connected backmixed flow reactors with volumes
PLEASE SOLVE IT CORRECTLY. DO NOT COPY.
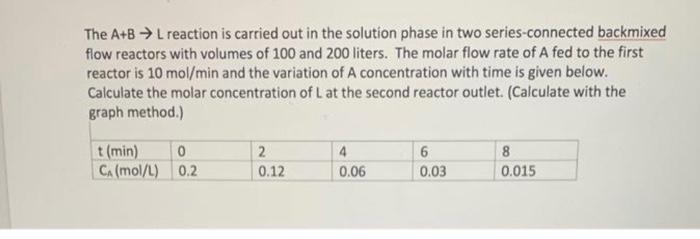
The A+BL reaction is carried out in the solution phase in two series-connected backmixed flow reactors with volumes of 100 and 200 liters. The molar flow rate of A fed to the first reactor is 10mol/min and the variation of A concentration with time is given below. Calculate the molar concentration of L at the second reactor outlet. (Calculate with the graph method.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


