Question: please solve it fast Q3 (a) Define buffer solution with suitable example. Discuss the importance of buffers in hair shampoos. (b) Consider the combustion of
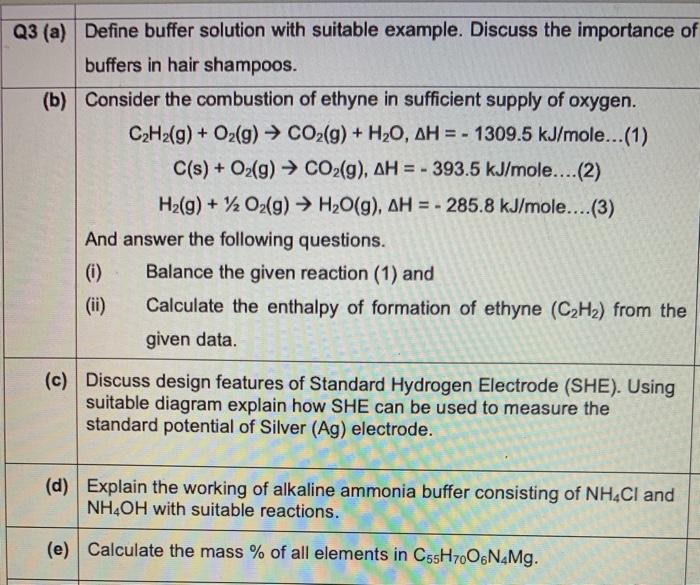
Q3 (a) Define buffer solution with suitable example. Discuss the importance of buffers in hair shampoos. (b) Consider the combustion of ethyne in sufficient supply of oxygen. C2H2(g) + O2(g) CO2(g) + H20, AH = - 1309.5 kJ/mole... (1) C(s) + O2(g) CO2(g), AH = - 393.5 kJ/mole....(2) H2(g) + 12 O2(g) H2O(g), AH = - 285.8 kJ/mole....(3) And answer the following questions. (i) Balance the given reaction (1) and (ii) Calculate the enthalpy of formation of ethyne (C2H2) from the given data. (c) Discuss design features of Standard Hydrogen Electrode (SHE). Using suitable diagram explain how SHE can be used to measure the standard potential of Silver (Ag) electrode. (d) Explain the working of alkaline ammonia buffer consisting of NH4Cl and NH4OH with suitable reactions. (e) Calculate the mass % of all elements in C55H700 N4Mg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


