Question: please solve it fast What is the worst moment with 10 5.17 O 7.500 C) 7.675 09.660 What is the value of P Mg2+ at
please solve it fast 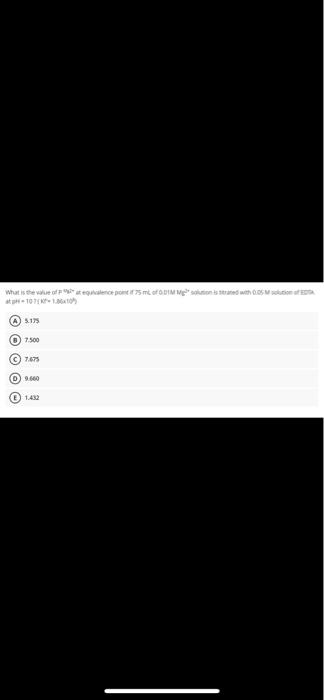


What is the worst moment with 10 5.17 O 7.500 C) 7.675 09.660 What is the value of P Mg2+ at equivalence point if 75 mL of 0.01M Mg2+ solution is titrated with 0.05 M solution of EDTA at pH = 10? (K'= 1.86x108) A 5.175 B 7.500 7.675 9.660 E 1.432
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


