Question: Please solve it in 1 hour i will upvote you Question 1 a) Clapeyron equation is widely used to determine the enthalpy/entropy change of vaporization.
Please solve it in 1 hour i will upvote you
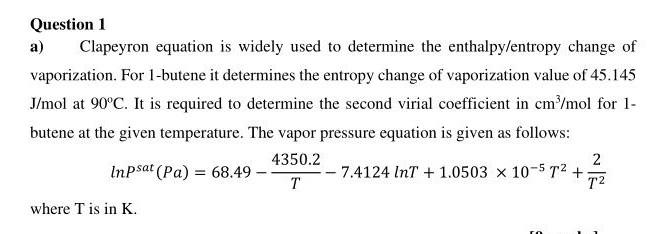
Question 1 a) Clapeyron equation is widely used to determine the enthalpy/entropy change of vaporization. For 1-butene it determines the entropy change of vaporization value of 45.145 J/mol at 90C. It is required to determine the second virial coefficient in cm /mol for 1- butene at the given temperature. The vapor pressure equation is given as follows: 4350.2 2 Inpsat (Pa) = 68.49 - 7.4124 InT + 1.0503 x 10-572 + T2 where T is in K
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


