Question: Please solve it in a clear way and using paper not using a computer Determine the standard heat of reaction for each of the following
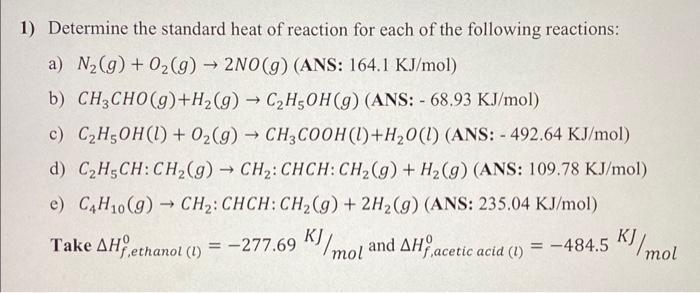
Determine the standard heat of reaction for each of the following reactions: a) N2(g)+O2(g)2NO(g) (ANS: 164.1KJ/mol) b) CH3CHO(g)+H2(g)C2H5OH(g) (ANS: 68.93KJ/mol) c) C2H5OH(l)+O2(g)CH3COOH(l)+H2O(l) (ANS: 492.64KJ/mol) d) C2H5CH:CH2(g)CH2:CHCH:CH2(g)+H2(g)( ANS: 109.78KJ/mol) e) C4H10(g)CH2:CHCH:CH2(g)+2H2(g)( ANS: 235.04KJ/mol) Take Hf,ethanol(l)0=277.69KJ/mol and Hf,aceticacid(l)0=484.5KJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


