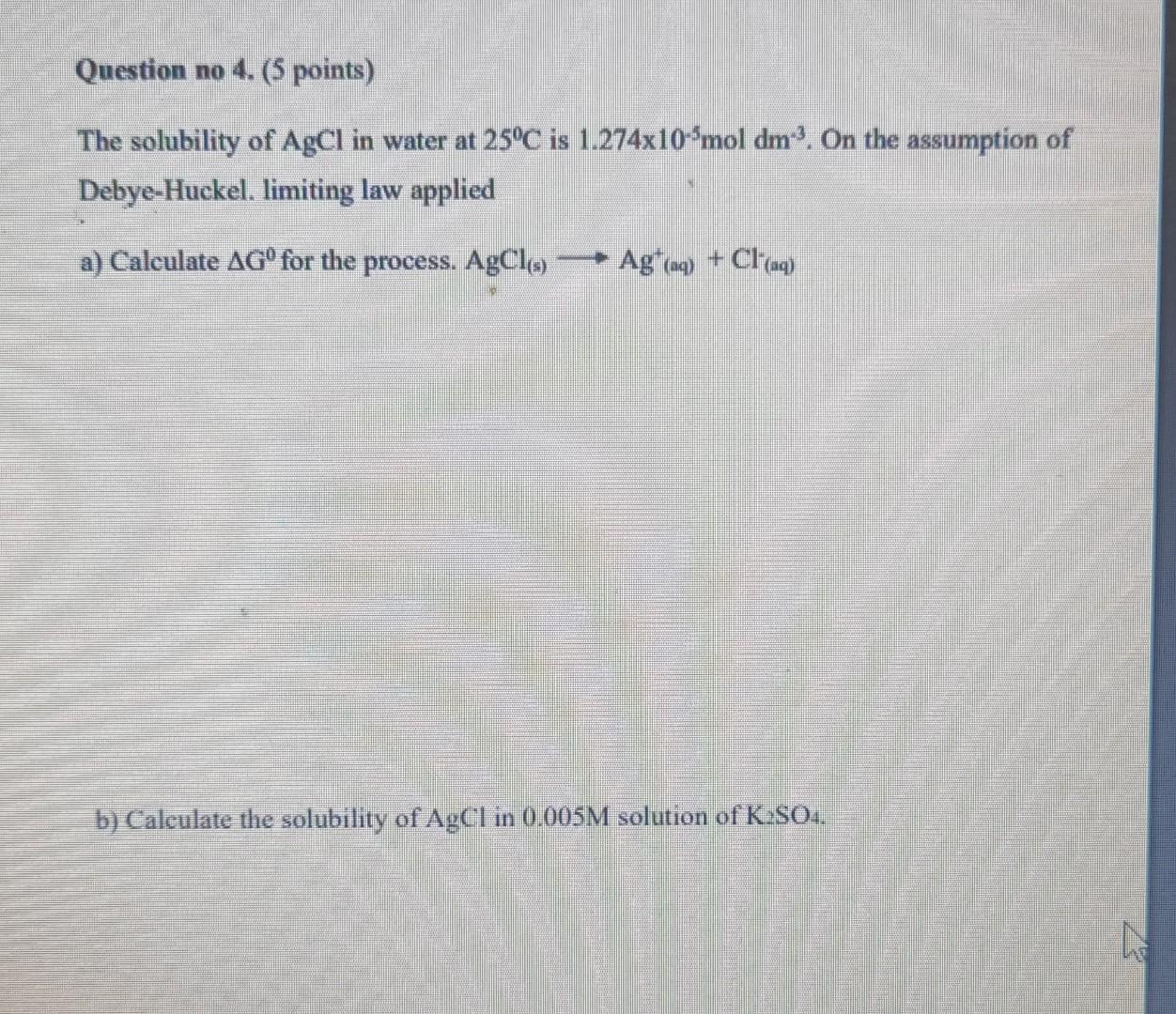
Question: please solve it quickly Question no 4. (5 points) The solubility of AgCl in water at 25C is 1.274x10'mol dm. On the assumption of Debye-Huckel.
please solve it quickly
Question no 4. (5 points) The solubility of AgCl in water at 25C is 1.274x10'mol dm. On the assumption of Debye-Huckel. limiting law applied a) Calculate AG for the process. AgCla) Ag+(aq) + Cl-aq) b) Calculate the solubility of AgCl in 0.005M solution of K S04. n
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


