Question: please solve it with a clear handwriting, not by computer 5) P-T diagram for water is given below. At point A there is 1kg water
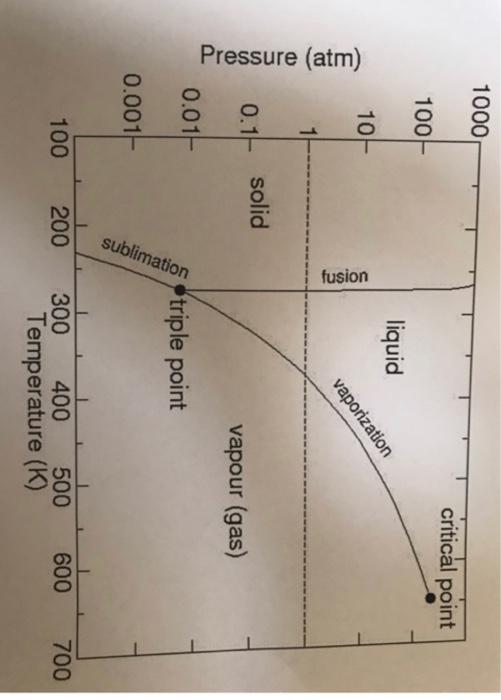
5) P-T diagram for water is given below. At point A there is 1kg water at 1atm and 20C. Keeping pressure constant, the temperature is increased up to the boiling temperature. Say it as point B. Then the pressure is increased up to 10atm keeping temperature constant (Point C). Then the temperature is increased keeping pressure constant up to the saturation point (Point D). Draw the four points in the graph and mention the existing phases, temperature, pressure and degree of freedom of every point and calculate volume of the system at every point. Take density of water as 1000 kg/m3 and quality ' q ' =0.5 where needed. Pressure (atm)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


