Question: Please solve part b using trapz in matlab. Thanks b) Do Problem 1-5 part b in your textbook (page 29), both CSTR and PFR. Use
Please solve part b using trapz in matlab. Thanks
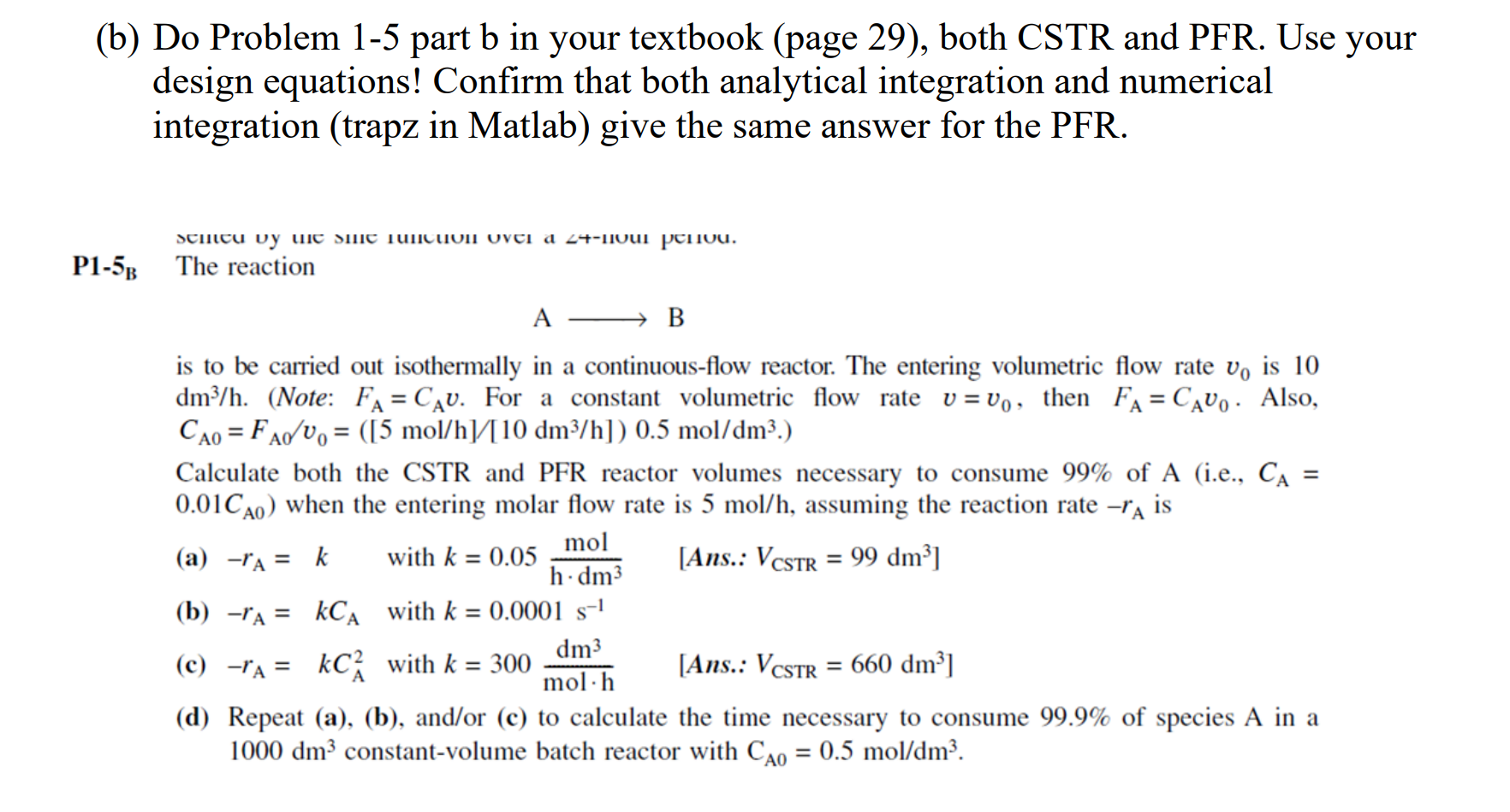
b) Do Problem 1-5 part b in your textbook (page 29), both CSTR and PFR. Use your design equations! Confirm that both analytical integration and numerical integration (trapz in Matlab) give the same answer for the PFR. 5B The reaction AB is to be carried out isothermally in a continuous-flow reactor. The entering volumetric flow rate v0 is 10 dm3/h. (Note: FA=CAv. For a constant volumetric flow rate v=v0, then FA=CAv0. Also, CA0=FA0/v0=([5mol/h]/[10dm3/h])0.5mol/dm3. ) Calculate both the CSTR and PFR reactor volumes necessary to consume 99% of A (i.e., CA= 0.01CA0 ) when the entering molar flow rate is 5mol/h, assuming the reaction rate rA is (a) rA=k with k=0.05hdm3mol [Ans.: VCSTR=99dm3] (b) rA=kCA with k=0.0001s1 (c) rA=kCA2 with k=300molhdm3 [Ans.: VCSTR=660dm3 ] (d) Repeat (a), (b), and/or (c) to calculate the time necessary to consume 99.9% of species A in a 1000dm3 constant-volume batch reactor with CA0=0.5mol/dm3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


