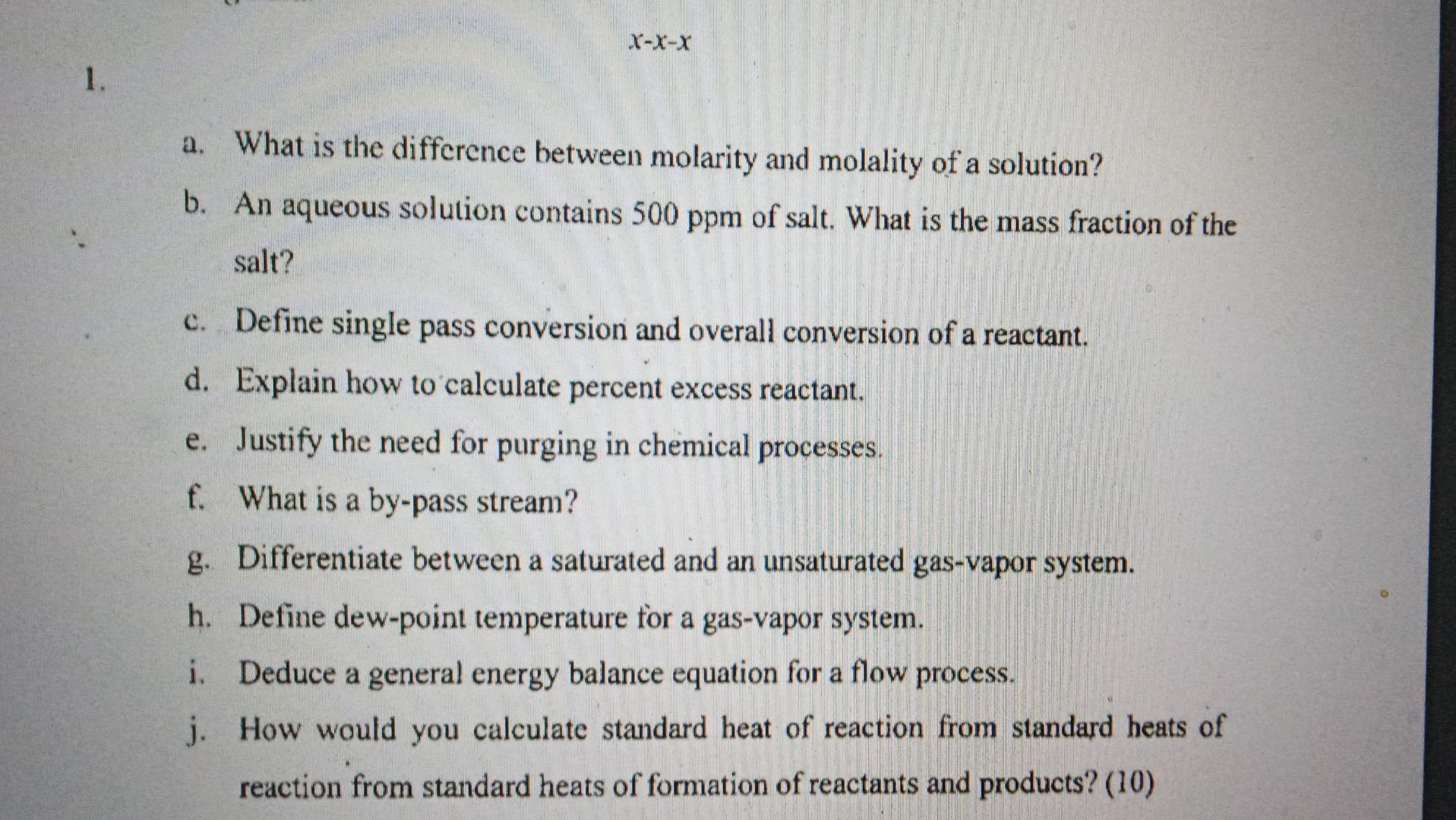
Question: please solve question no. 1 all parts . please solve fast. X-x-X 1. a. What is the difference between molarity and molality of a solution?
please solve question no. 1 all parts . please solve fast.
X-x-X 1. a. What is the difference between molarity and molality of a solution? b. An aqueous solution contains 500 ppm of salt. What is the mass fraction of the salt? c. Define single pass conversion and overall conversion of a reactant. d. Explain how to calculate percent excess reactant. e. Justify the need for purging in chemical processes. f. What is a by-pass stream? g. Differentiate between a saturated and an unsaturated gas-vapor system. h. Define dew-point temperature for a gas-vapor system. i. Deduce a general energy balance equation for a flow process. j. How would you calculate standard heat of reaction from standard heats of reaction from standard heats of formation of reactants and products? (10) a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


