Question: please solve step by step Notes: A/l answers to questions require an explanation. Show your work and always check units and sig figs. on calculations
please solve step by step 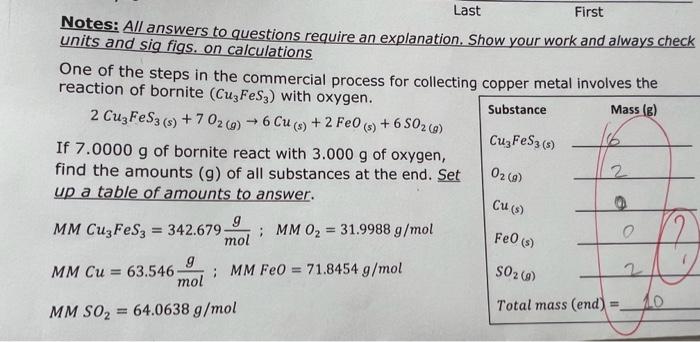

Notes: A/l answers to questions require an explanation. Show your work and always check units and sig figs. on calculations One of the steps in the commercial process for collecting copper metal involves the reaction of bornite (Cu3FeS3) with oxygen. 2Cu3FeS3(s)+7O2(g)6Cu(s)+2FeO(s)+6SO2(g) If 7.0000g of bornite react with 3.000g of oxygen, find the amounts (g) of all substances at the end. Set up a table of amounts to answer. MMCu3FeS3=342.679molg;MMO2=31.9988g/molMMCu=63.546molg;MMFeO=71.8454g/molMMSO2=64.0638g/mol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


