Question: Please solve step by step. Thanks Du. A constant-mole batch distillation is used to change from a pure n-butanol solvent to a solvent that is
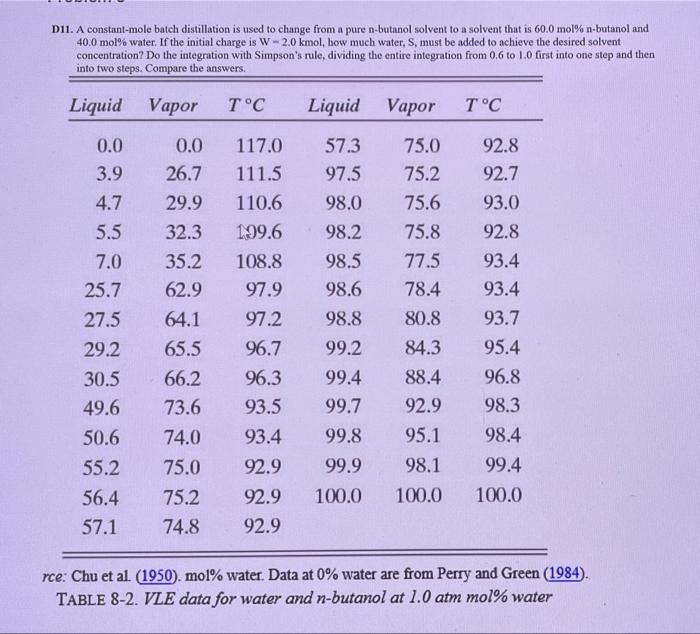
Du. A constant-mole batch distillation is used to change from a pure n-butanol solvent to a solvent that is 60.0 mol% n-butanol and 40.0 mol% water. If the initial charge is W - 2.0 kmol, how much water, S, must be added to achieve the desired solvent concentration? Do the integration with Simpson's rule, dividing the entire integration from 0.6 to 10 first into one step and then into two steps. Compare the answers. Liquid Vapor TC Liquid Vapor TC 98.2 0.0 3.9 4.7 5.5 7.0 25.7 27.5 29.2 0.0 26.7 29.9 32.3 35.2 62.9 64.1 65.5 66.2 73.6 74.0 75.0 75.2 74.8 117.0 57.3 111.5 97.5 110.6 98.0 199.6 108.8 98.5 97.9 98.6 97.2 98.8 96.7 99.2 96.3 99.4 93.5 99.7 93.4 99.8 92.9 99.9 92.9 100.0 92.9 75.0 92.8 75.2 92.7 75.6 93.0 75.8 92.8 77.5 93.4 78.4 93.4 80.8 93.7 84.3 95.4 88.4 96.8 92.9 98.3 95.1 98.4 98.1 99.4 100.0 100.0 30.5 49.6 50.6 55.2 56.4 57.1 rce: Chu et al. (1950). mol% water. Data at 0% water are from Perry and Green (1984) TABLE 8-2. VLE data for water and n-butanol at 1.0 atm mol% water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


