Question: please solve table3 and the calculations. Data Tables Table 1: Measured Values for the density ID of the unknown metal Tin (Su) Mass of the
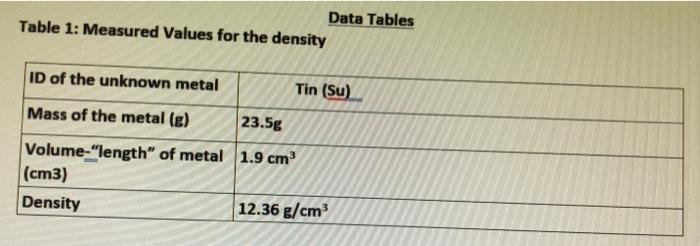
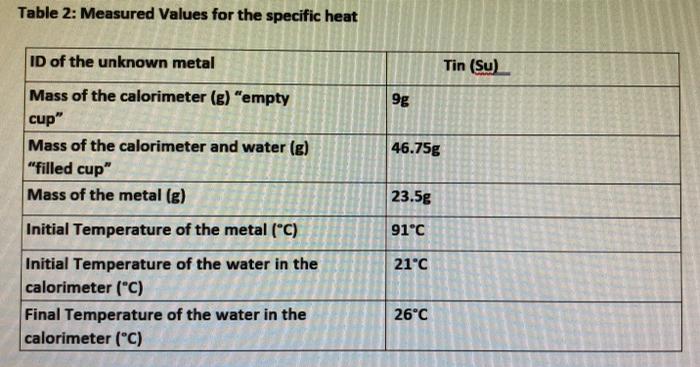
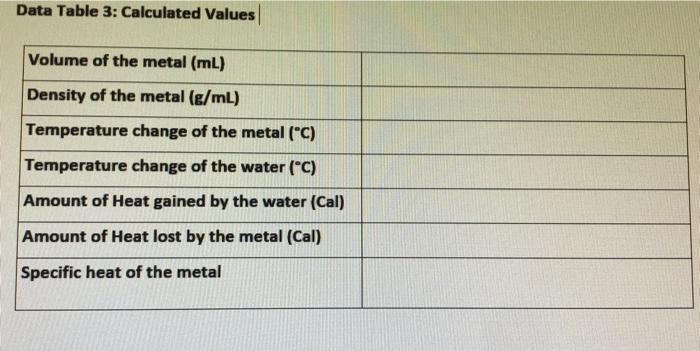

Data Tables Table 1: Measured Values for the density ID of the unknown metal Tin (Su) Mass of the metal (g) 23.5g Volume-"length" of metal 1.9 cm (cm3) Density 12.36 g/cm Table 2: Measured Values for the specific heat ID of the unknown metal Tin (Su) 9g Mass of the calorimeter (e) "empty cup" Mass of the calorimeter and water (g) "filled cup" Mass of the metal (g) 46.75g 23.5g Initial Temperature of the metal (C) 91C 21C Initial Temperature of the water in the calorimeter ("C) Final Temperature of the water in the calorimeter (C) 26C Data Table 3: Calculated Values Volume of the metal (mL) Density of the metal (g/mL) Temperature change of the metal ("C) Temperature change of the water (C) Amount of Heat gained by the water (Cal) Amount of Heat lost by the metal (Cal) Specific heat of the metal Calculations: Show your calculations below and report your answer in Data table 1.) 1.)Calculate the density of the unknown metal. I 2.) Determine the amount of heat in J and Cal gained by the water. 3.) How much energy (in J and Cal) was "lost" by the piece of metal? Explain your answer. 4.) Determine the specific heat of the metal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


