Question: please solve the calculations part. Also, there are some tables you can use. Performance of Single Effect Evaporator. At time t : - The

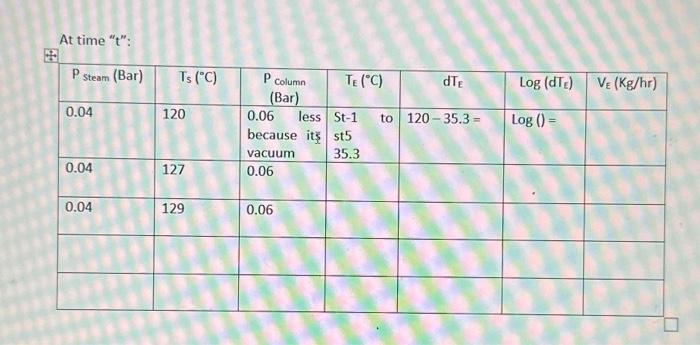

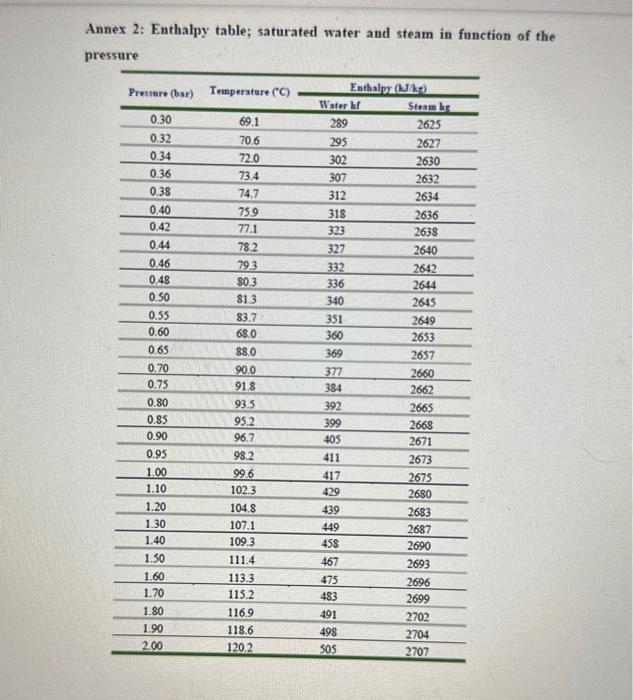
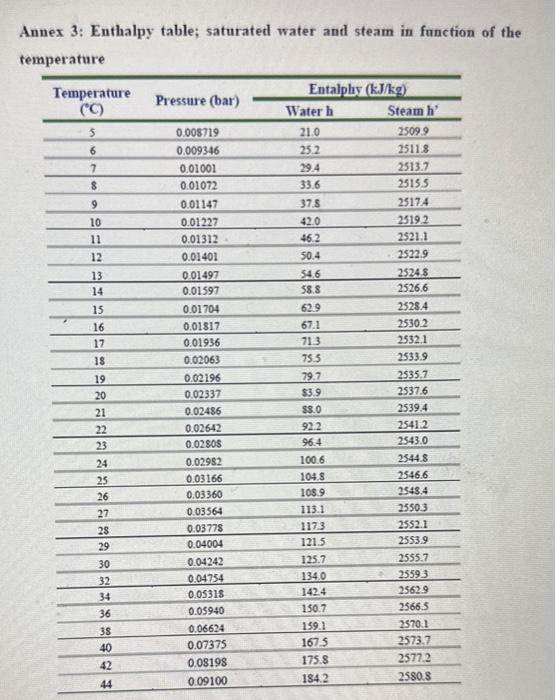
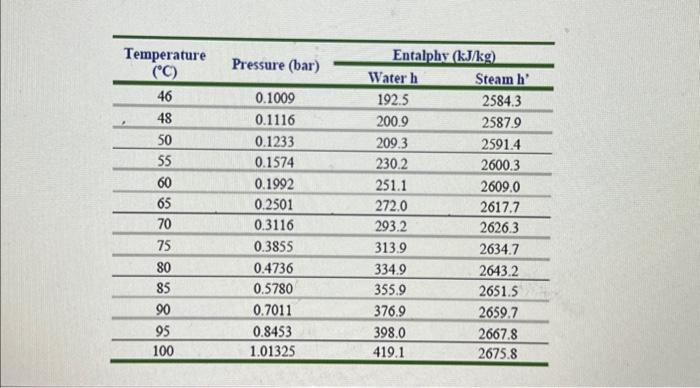
Performance of Single Effect Evaporator. At time " t ": - The evaporation speed, VE is given by the equation: VE=Q/H in kg/h units - Where, Q= heat transfer velocity (kJ/h) - H = latent heat of water vaporization at the working pressure of the system (kJ/Kg) - The heat transfer velocity, Q=UEAEdTE - Where, UE= global heat transfer coefficient (kJ/(m2hC) ) (find at TE). - AE= Heat transmission surface (m2) - dTE= Temp. of steam at pressure P1(Ts) - Boiling point of water at the pressure of the system (TE) - Evaporator heating area =0.122m2 - Immersion heating element power =400kWh=86.4kJ - Dosing pump flow control =0.43ml/ pulse - Specific density of 4% wt. sugar solution =1.014 at 20C Annex 2: Enthalpy table; saturated water and steam in function of the pressure Annex 3: Enthalpy table; saturated water and steam in function of the temnorature \begin{tabular}{cccc} \hline Temperature & & \multicolumn{2}{c}{ Entalphy (kJ/kg) } \\ \cline { 3 - 4 }(C) & Pressure (bar) & Water h & Steam h' \\ \hline 46 & 0.1009 & 192.5 & 2584.3 \\ \hline 48 & 0.1116 & 200.9 & 2587.9 \\ \hline 50 & 0.1233 & 209.3 & 2591.4 \\ \hline 55 & 0.1574 & 230.2 & 2600.3 \\ \hline 60 & 0.1992 & 251.1 & 2609.0 \\ \hline 65 & 0.2501 & 272.0 & 2617.7 \\ \hline 70 & 0.3116 & 293.2 & 2626.3 \\ \hline 75 & 0.3855 & 313.9 & 2634.7 \\ \hline 80 & 0.4736 & 334.9 & 2643.2 \\ \hline 85 & 0.5780 & 355.9 & 2651.5 \\ \hline 90 & 0.7011 & 376.9 & 2659.7 \\ \hline 95 & 0.8453 & 398.0 & 2667.8 \\ \hline 100 & 1.01325 & 419.1 & 2675.8 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


