Question: Please, solve the problem in full explanation and full calculations: A mixture of air and a hydrocarbon fuel whose average composition is indicated by CH1.9
Please, solve the problem in full explanation and full calculations:
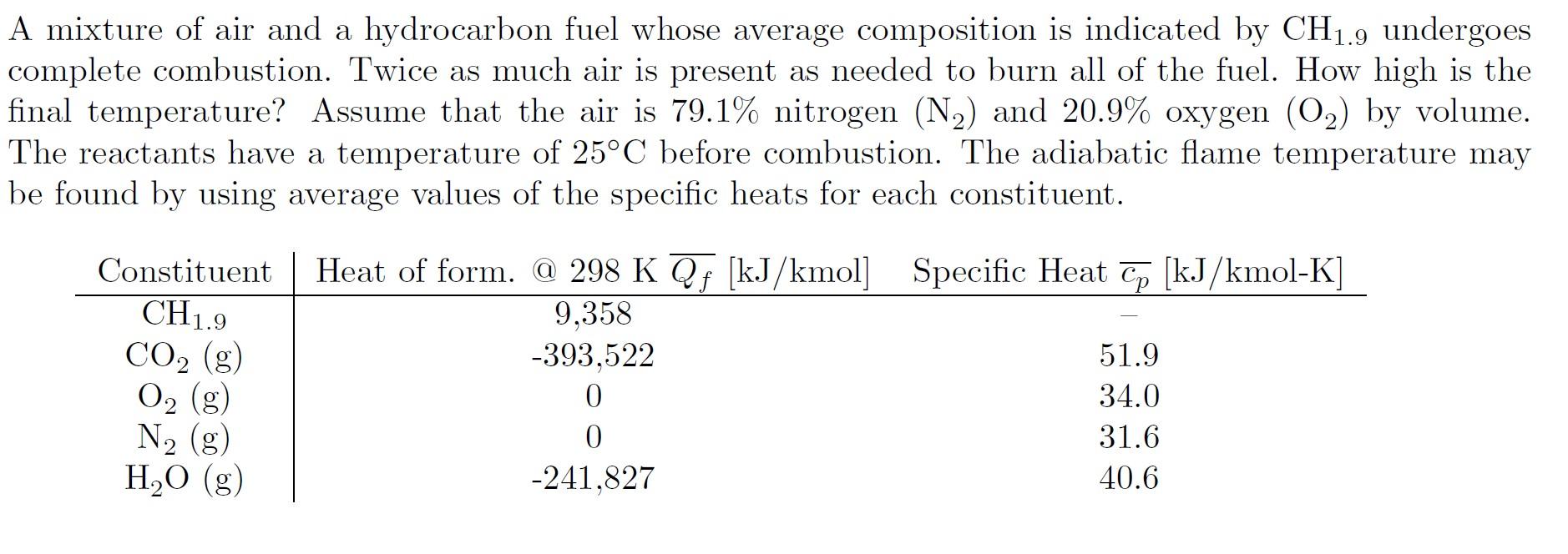
A mixture of air and a hydrocarbon fuel whose average composition is indicated by CH1.9 undergoes complete combustion. Twice as much air is present as needed to burn all of the fuel. How high is the final temperature? Assume that the air is 79.1% nitrogen (N2) and 20.9% oxygen (O2) by volume. The reactants have a temperature of 25C before combustion. The adiabatic flame temperature may be found by using average values of the specific heats for each constituent. A mixture of air and a hydrocarbon fuel whose average composition is indicated by CH1.9 undergoes complete combustion. Twice as much air is present as needed to burn all of the fuel. How high is the final temperature? Assume that the air is 79.1% nitrogen (N2) and 20.9% oxygen (O2) by volume. The reactants have a temperature of 25C before combustion. The adiabatic flame temperature may be found by using average values of the specific heats for each constituent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


