Question: Please, solve the problem in full explanation and full details: Assuming complete combustion with oxygen, O2, study the combustion of methane, CH4, initially at 298.15K
Please, solve the problem in full explanation and full details: 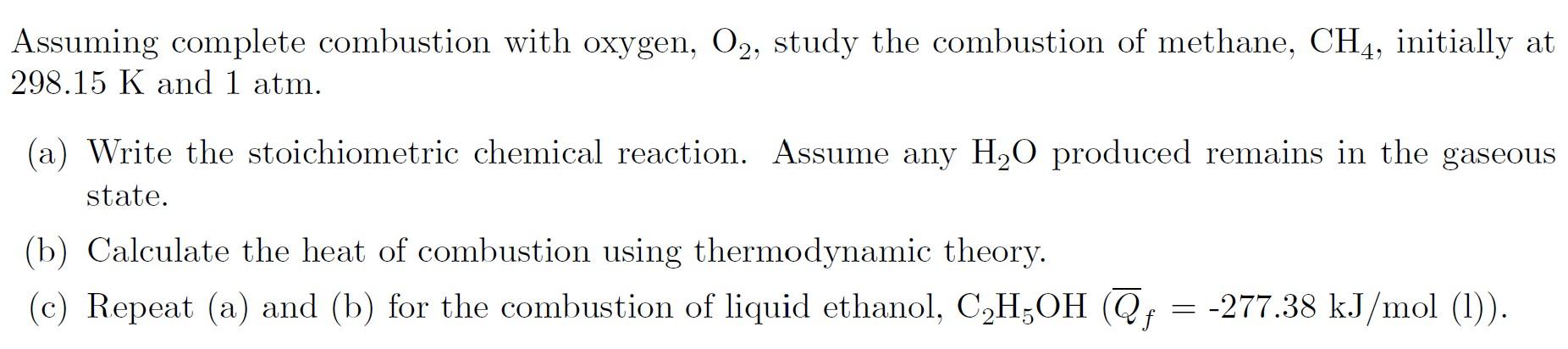
Assuming complete combustion with oxygen, O2, study the combustion of methane, CH4, initially at 298.15K and 1atm. (a) Write the stoichiometric chemical reaction. Assume any H2O produced remains in the gaseous state. (b) Calculate the heat of combustion using thermodynamic theory. (c) Repeat (a) and (b) for the combustion of liquid ethanol, C2H5OH(Qf=277.38kJ/mol (l))
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


